Cyanine compound, composition containing same and application in cell detection thereof
A technology of compounds and compositions, applied in the field of fluorescent dyes, can solve the problems of unsatisfactory accuracy of nucleated red blood cell counting, basophil differential counting, NRBC counting and other problems, and achieve good cell classification effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
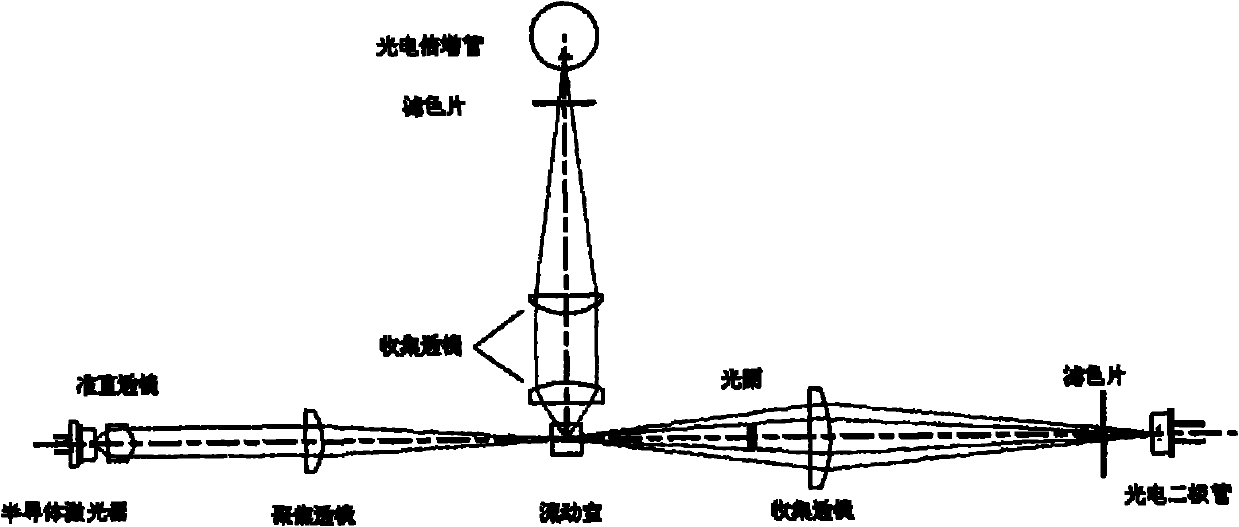
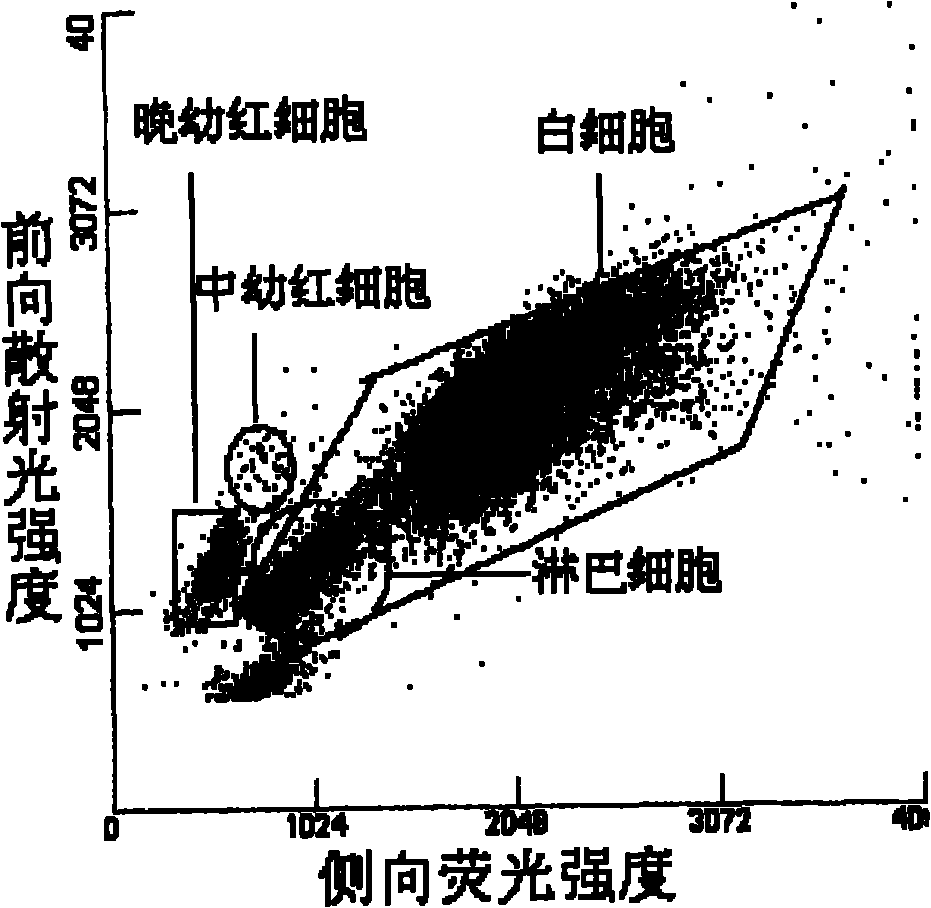
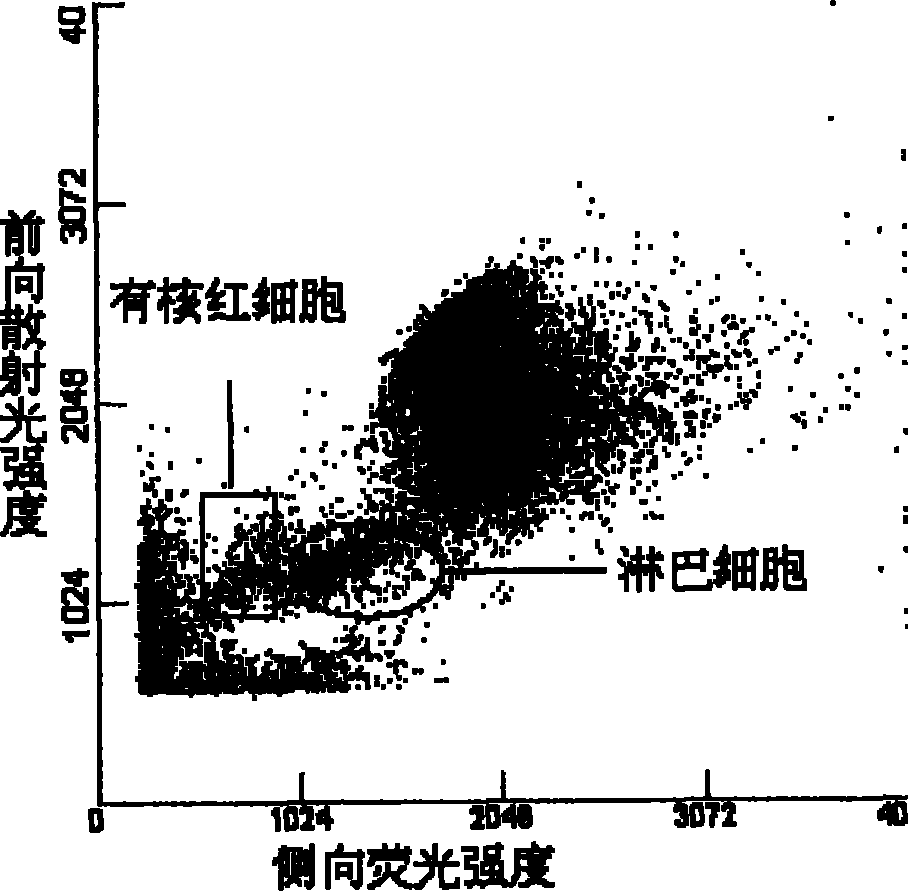
Image
Examples
preparation example Construction
[0079] The preparation method of the compound of the present invention
[0080]The compounds of the present invention can be synthesized by general methods well known in the art. Specifically, some intermediates of the compounds of the present invention can be synthesized through the following schemes.
[0081] By unsubstituted or substituted compound of formula IV as raw material, respectively with formula R 1 X or R 3 Halide reaction of X (X is F, Cl, Br or I):
[0082]
[0083] Obtain the quaternary ammonium salt intermediate of formula V and formula VI:
[0084]
[0085] where R 1 , R 2 , R 3 , X and A1 rings are defined as described for compounds of formula I.
[0086] For example, the corresponding quaternary ammonium salt intermediate is obtained by the following reaction.
[0087]
[0088] Then, the prepared quaternary ammonium salt intermediate is condensed with a linking molecule such as squarylium to obtain a compound of formula I:
[0089]
...
Embodiment 1
[0157] Preparation of Compound 1
[0158]
[0159] Add 0.1mol 2,3,3-trimethylindole, 0.12mol allyl bromide and 25mL acetonitrile into a 100mL three-necked flask equipped with a reflux condenser and a magnetic heating stirrer as solvents. Protected from light, the reaction was refluxed for 24 hours under the protection of argon. After the reaction was completed, part of the solvent was distilled off under reduced pressure, and an appropriate amount of petroleum ether was added thereto, and the product was precipitated by ultrasonic vibration to obtain a brownish-red oil, which was directly used in the next reaction.
[0160] Take 0.01mol of the brown-red product, 0.005mol of squaraine, 8mL of benzene, 6mL of n-butanol, and 6mL of pyridine as solvents and put them into a 50mL three-neck flask together, stir and heat to reflux under the protection of argon, and stop the reaction after 6 hours. After cooling to room temperature, excess diethyl ether was added to precipitate th...
Embodiment 2
[0163] Preparation of compound 2
[0164]
[0165] Add 0.1mol 2,3,3-trimethylindole, 0.12mol butyl bromide and 20mL toluene to a 100mL three-necked flask equipped with a reflux condenser and a magnetic heating stirrer as a solvent, avoid light, and protect it under argon Reflux for 24 hours. After the reaction was completed, part of the solvent was distilled off under reduced pressure, and an appropriate amount of petroleum ether was added thereto, and the product was precipitated by ultrasonic vibration to obtain a brownish-red lump, which was directly used in the next reaction.
[0166] Take 0.01mol of the brown-red product, 0.005mol of squaraine, 8mL of toluene, 6mL of n-butanol, and 6mL of pyridine as solvents and put them together into a 50mL three-neck flask, stir and heat to reflux under the protection of argon, and stop the reaction after 7 hours. After cooling to room temperature, excess diethyl ether was added to precipitate the product, filtered, washed with an ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com