Polyethylene glycol modified protein separating and purifying method
A polyethylene glycol, separation and purification technology, applied in the preparation methods of peptides, chemical instruments and methods, organic chemistry, etc., can solve the problems of troublesome resin cleaning and regeneration, pollution, affecting the service life of resins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Separation and purification of PEGylated recombinant human granulocyte-stimulating factor
[0043] 1.1. Pegylation modification of recombinant human granulocyte stimulating factor
[0044] The modification process refers to Chinese patent 95191454.5. Take a tube containing 140mg of mPEG-ALD (7.09μmol) with a molecular weight of 20KDa, add 20mg of rhG-CSF solution (1.06 μmol). After the mPEG-ALD was dissolved, 0.082ml of 1M sodium cyanoborocyanide was added, and the reaction was stirred at 4°C for 16h. After the reaction was completed, the reaction solution was first diluted to 50 ml with 1 mM HCl, the protein concentration was 0.5 mg / ml, and then the pH of the reaction solution was adjusted to 4.0 with 1 M HCl.
[0045] 1.2. MacroCap SP ion exchange column chromatography
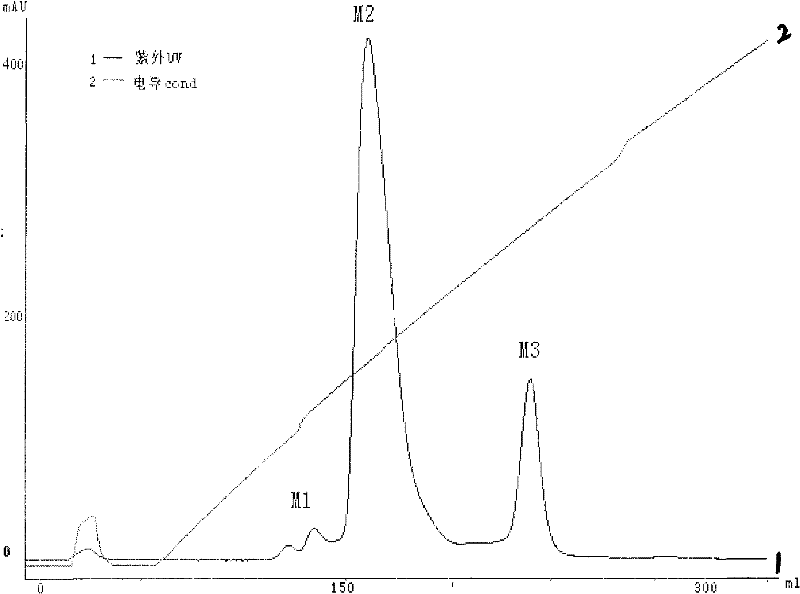
[0046]Purification was carried out by MacroCap SP cation exchange column chromatography. The isoelectric point of polyethylene glycol recombinant human granulocyte-stimulating factor is...
Embodiment 2
[0048] Example 2 Separation and purification of PEGylated recombinant human granulocyte-stimulating factor
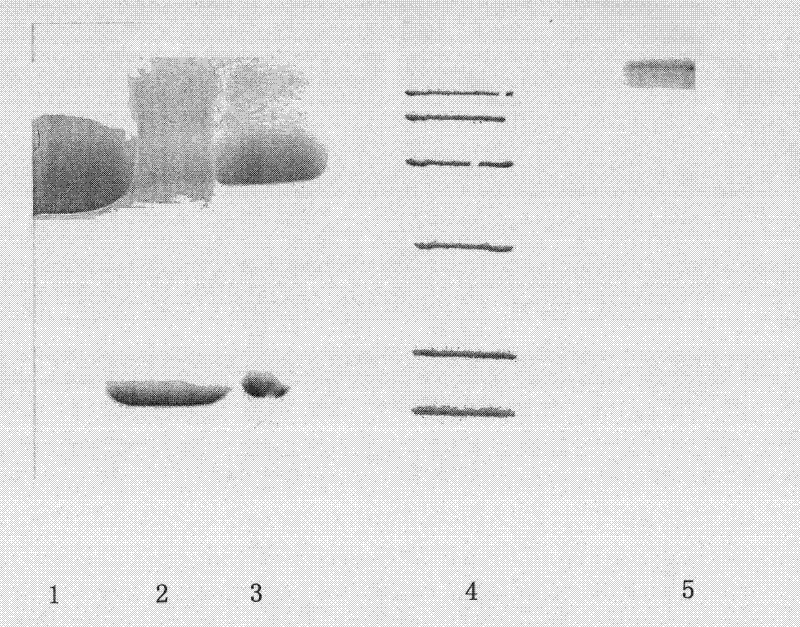
[0049] The elution condition is to first elute multiple PEG-modified rhG-CSF with 15% 0.5mol / L NaCl solution, and then elute the single-modified target peak with 30% 0.5mol / L NaCl solution. Other conditions are basically the same as those in practice. Similar to Example 1, the electrophoresis results indicated that the purified target peaks obtained more than 90%.
[0050] Further separation and purification of the purified sample of embodiment 3 embodiment 1
[0051] The specific experimental process is similar to the initial purification process. The elution condition is to first elute the impurity protein with 10% 0.5mol / L NaCl solution, and then elute the single-modified target peak with 35% 0.5mol / L NaCl solution. ( image 3 ).
Embodiment 4
[0052] Example 4 MacroCap SP Ion Exchange Chromatography Separation and Purification of PEG-exendin-4
[0053] 4.1. Preparation of PEG-Exendin-4 conjugates
[0054] Add 1M sodium acetate (pH5.0~pH6.0) buffer solution and water for injection to the Exendin-4 solution stored in the refrigerator at 2~8℃ to make the concentration of Exendin-4 2.5~5mg / ml, and the final concentration of sodium acetate is 0.1 M, add 2.5-5 times molar ratio of mPEG-ALD, after the mPEG-ALD dissolves, add a final concentration of 10-20mM NaCNBH3, and react for 16-24h. The modification rate of the modified sample should not be less than 70% by SEC-HPLC analysis.
[0055] 4.2. Separation and purification of PEG-exendin-4 by MacroCap SP ion exchange chromatography
[0056] MacroCap SP ion-exchange chromatography medium was selected, and the chromatography column was equilibrated with equilibration buffer (20mmol / L NaAc, pH4.0). Dilute 100ml of the modified reaction sample 3 times with purified water, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com