CD19-CAR-T cell carrying iCasp9 suicide gene and use thereof
A gene and lymphocyte technology, applied in the fields of genetic engineering and cell biology, can solve problems such as side effects and patient death, and achieve the effect of increasing safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Design of CD19CAR containing iCasp9 suicide gene and construction of expression vector
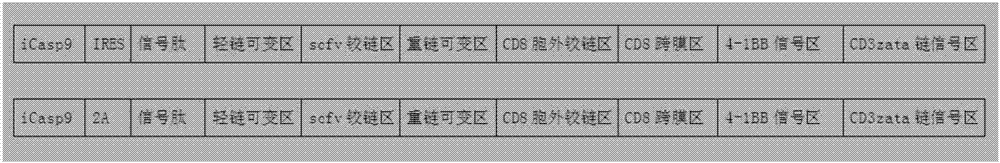
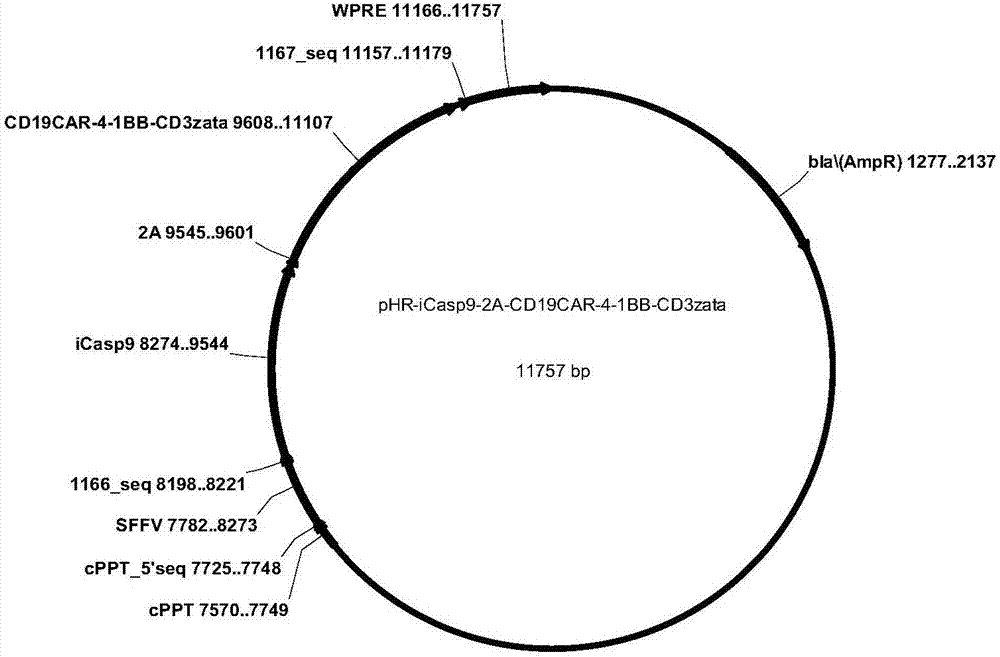
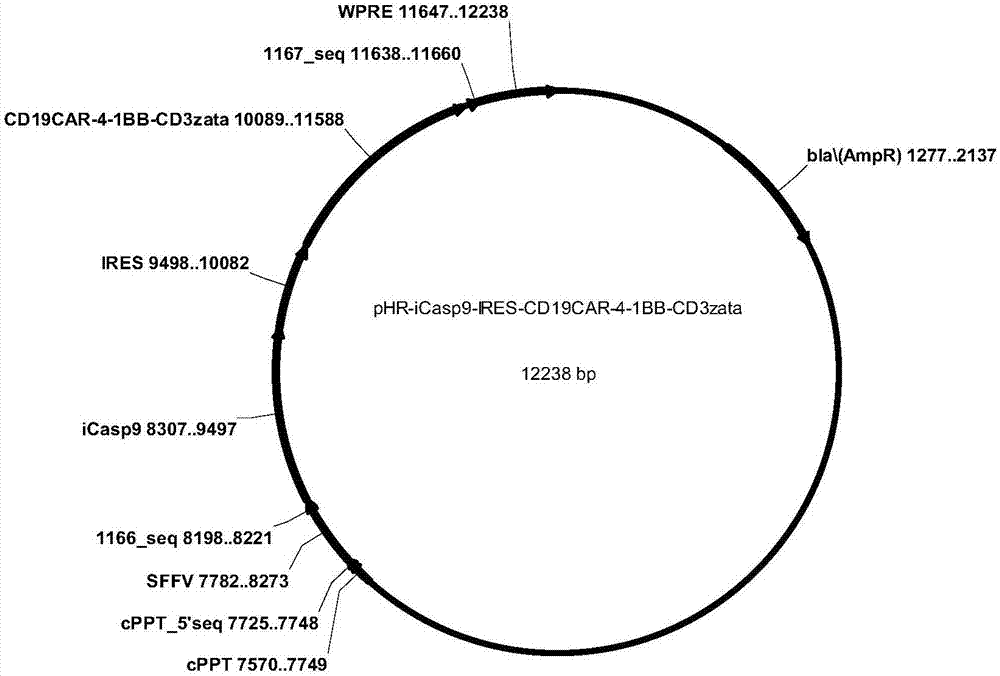
[0056]According to the different construction strategies of iCasp9 and CD19CAR dual expression vectors, two CD19CARs containing iCasp9 suicide gene were designed and constructed, named pHR-iCasp9-2A-CD19CAR-4-1BB-CD3ζ and pHR-iCasp9-IRES-CD19CAR-4 respectively -1BB-CD3ζ, wherein the amino acid residue sequence expressed by pHR-iCasp9-2A-CD19CAR-4-1BB-CD3ζ is as shown in SEQID NO:17, expressed by pHR-iCasp9-IRES-CD19CAR-4-1BB-CD3ζ The amino acid residue sequence is shown in SEQID NO: 19, and the pattern is shown in figure 1 As shown, the structure diagram is as follows figure 2 , image 3 shown.
Embodiment 2
[0057] Example 2: Packaging and concentration of lentivirus
[0058] The lentiviral expression vector carrying the gene of interest (pHR-iCasp9-2A-CD19CAR-4-1BB-CD3ζ, pHR-iCasp9-IRES-CD19CAR-4-1BB-CD3ζ constructed in Example 1), pCMV vector and pMD. 2G vectors were mixed and transfected into 293FT cells, replaced with complete medium for culture 6-8 hours after transfection, collected the culture medium after 48 hours, retained the supernatant after centrifugation and filtered the supernatant with a 0.45 μm filter, and retained the filtrate. The filtrate is the solution of the recombinant lentivirus.
[0059] Lentivirus concentration was carried out according to the instructions of Lenti-XTM Concentrator (takara, cat: 631231).
Embodiment 3
[0060] Embodiment 3: Verification of iCasp9 / CID suicide gene system
[0061] 1. Preparation of K562 cells expressing lentiviral vector
[0062] Resuspend the cells in 1640 medium to 1x106 / mL. Add lentivirus. 37°C, 5% CO 2 Cultivate in an incubator for 6-8 hours, and replace the culture medium by centrifugation with fresh K562 cell proliferation culture medium. Add fresh K562 cell proliferation medium every 2-3 days to maintain the cell density at 0.5x10 6 / mL or so. After 48 hours of virus infection, the proportion of CAR-positive cells was detected by flow cytometry (Fluorescein (FITC) AffiniPure Goat Anti-Mouse IgG, F(ab')2 fragment specific, jackson immunoresearch, cat: 115-095-006).
[0063] The result is as Figure 4 As shown, the proportion of CD19CAR-K562 cells containing the iCasp9 suicide gene is 34%, indicating that the CD19CAR-K562 cells containing the iCasp9 suicide gene have been successfully obtained and named as iCasp9-CD19CAR-K562 cells. As effector cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com