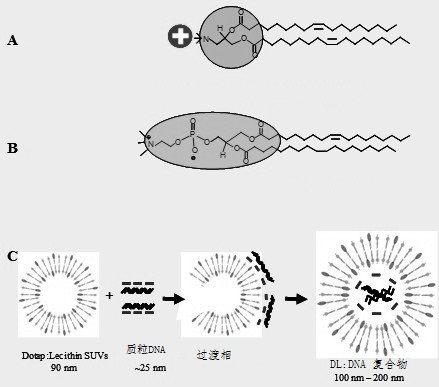
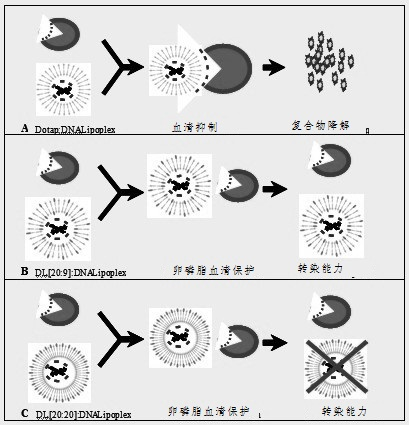
Tumor targeted therapeutic drug carrier as well as preparation method and application thereof
A treatment drug and tumor-targeting technology, applied in biochemical equipment and methods, anti-tumor drugs, and other methods of inserting foreign genetic materials, etc., can solve problems such as difficult control, random carrier loading, and poor therapeutic effect. Achieve large packaging volume, good blood stability, and good tumor treatment targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
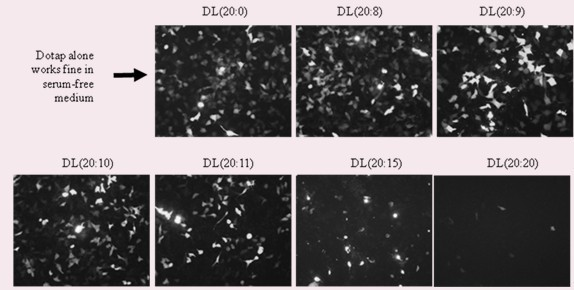
[0065] Example 1: The transfectivity experiment of DOTAP and lecithin in serum-free medium to H1299 cells in different ratios image 3 As shown, the transfection ability of DOTAP decreased with the increase of lecithin addition. 1 μg / μl of pFCB-eGFP was mixed with equal volumes of DL liposomes at different Dotap:Lecithin molar ratios. H1299 cells were inoculated into 12-well culture plates and treated with Opti-MEM culture medium containing 2.5 μl DL:DNA complex for 4 hours for transfection, and then replaced Opti-MEM with culture medium RPMI containing 10% fetal bovine serum, and culture plates were placed in The incubator was incubated for another 48 hours, observed and photographed with a fluorescence microscope at a magnification of 200, and obtained image 3 the result of.
Embodiment 2
[0066] Example 2: Different transfection efficiencies of complexes formed by DOTAP and lecithin and DNA under high or low serum concentration conditions
[0067] Such as Figure 4 As indicated, H1299 cells were seeded into 12-well culture plates at 37°C, 5% CO 2 Incubate overnight. When the cells grow to 30% saturation, the cells are treated with 1ml culture medium containing 1μl, 2.5μl or 5μl DL:pFCB-eGFP, the culture medium is RPMI-1640 containing 10% fetal bovine serum (FBS) or 1ml 100% FBS. The treated cells were gently shaken by hand every 30 minutes. After 4 hours, the transfection culture solution was replaced with 1ml RPMI-1640+10% FBS culture solution, and after 48 hours of cultivation, the photos were observed with a fluorescence microscope at a magnification of 200. 8mM liposomes with different Dotap: lecithin molar ratios and the same A volume of 1 μg / μl pFCB-eGFP plasmid DNA was bound. The photographs shown here are for Dotap:lecithin molar ratios of 20:8 to ...
Embodiment 3
[0068] Example 3: The relationship between the transfection efficiency and the particle size of the complex formed by the carrier formed by DOTAP and lecithin with a molar ratio of 20:9 and DNA
[0069] Such as Figure 5 As shown, the transfection efficiency of the complex is closely related to the particle size of the complex. A DNA mixture containing 0.2 μg / μl of pFCB-eGFP and 1 μg / μl of pFCB-Luc was mixed with an equal volume of DL [20:9]. The complexes were then filtered through filters with a pore size of 1 μm and 0.45 μm., labeled 1 μm and 0.45 μm. A DNA mixture containing 0.2 μg / μl pFCB-eGFP and 0.6 μg / μl pFCB-Luc was mixed with an equal volume of DL [20:9]. The complexes were then filtered through filters with a pore size of 0.2 μm and 0.1 μm., labeled 0.2 μm and 0.1 μm. H1299 cells were inoculated into 12-well culture plates, and the transfection operation was performed when the cells reached 30% saturation within 24 hours. Transfections were performed with 2 μl a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com