Recombinant slow virus vector, recombinant slow virus and stem cell containing recombinant slow virus
A technology of recombinant lentivirus and lentiviral vector, applied in the field of medical molecular biology, can solve problems such as easy malignant transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
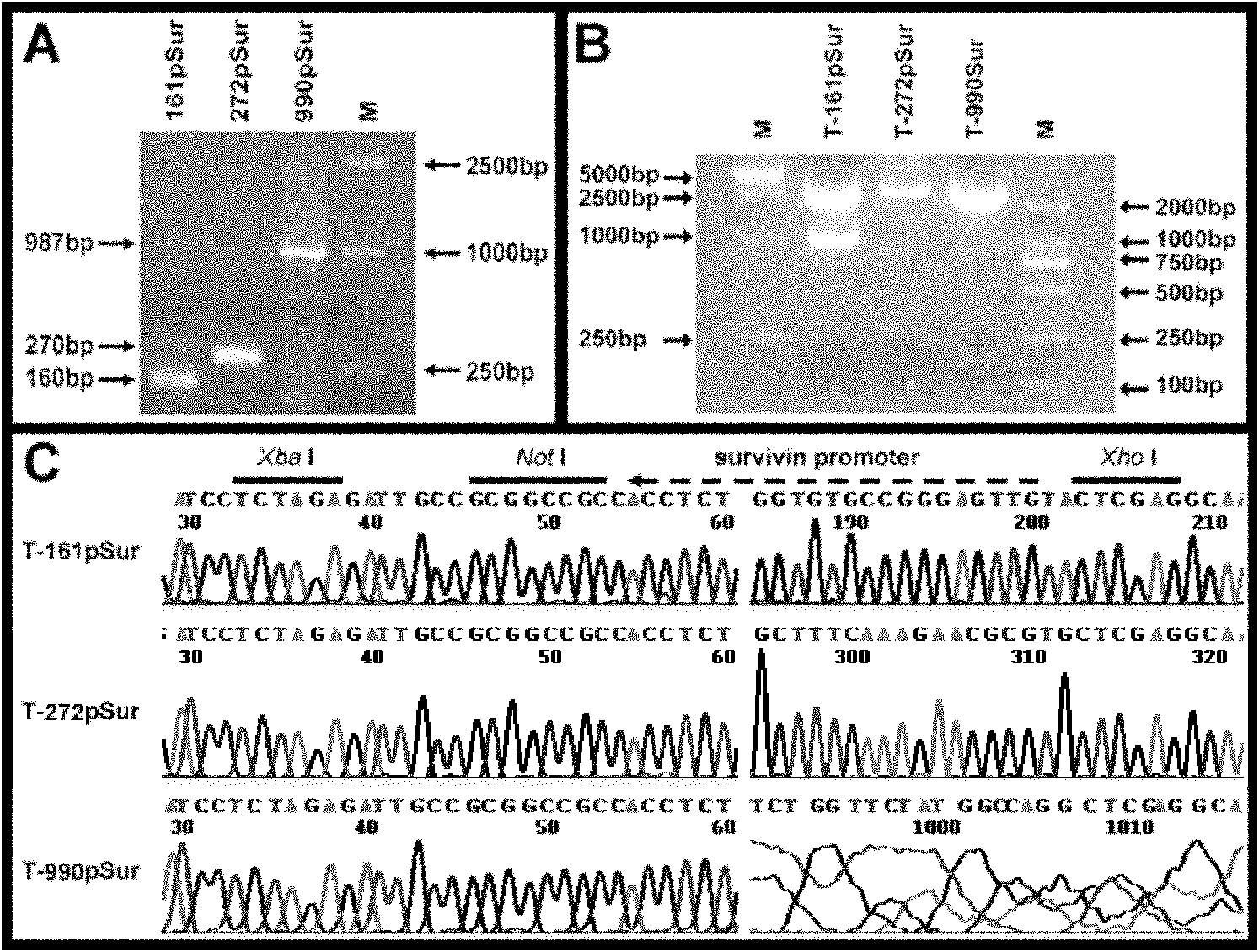
[0069] Example 1: Construction of recombinant lentiviral vector.
[0070] Sources of biological materials involved in the present invention:
[0071] T-apoptin carrier: a gift from researcher Yang Bing of Beijing Academy of Agriculture and Forestry, commercially available
[0072] FG-12: Gift from Prof. David Baltimore (David Geffen School of Medicine, University of California, Los Angeles, USA). The vector lacks E1 and E3 regions, and can insert large fragments of foreign genes. In addition to the above advantages, it also comes with an EGFP tracer gene, which can be used to trace the distribution of transplanted stem cells in vivo. See Figure 14 .
[0073] Auxiliary packaging plasmid: Invitrogen's Virapower system.
[0074] 293FT cells: purchased from Shanghai Institute of Cell Biology.
[0075] The above biological materials are preserved in this laboratory and can be released to the public for verification experiments.
Embodiment 2
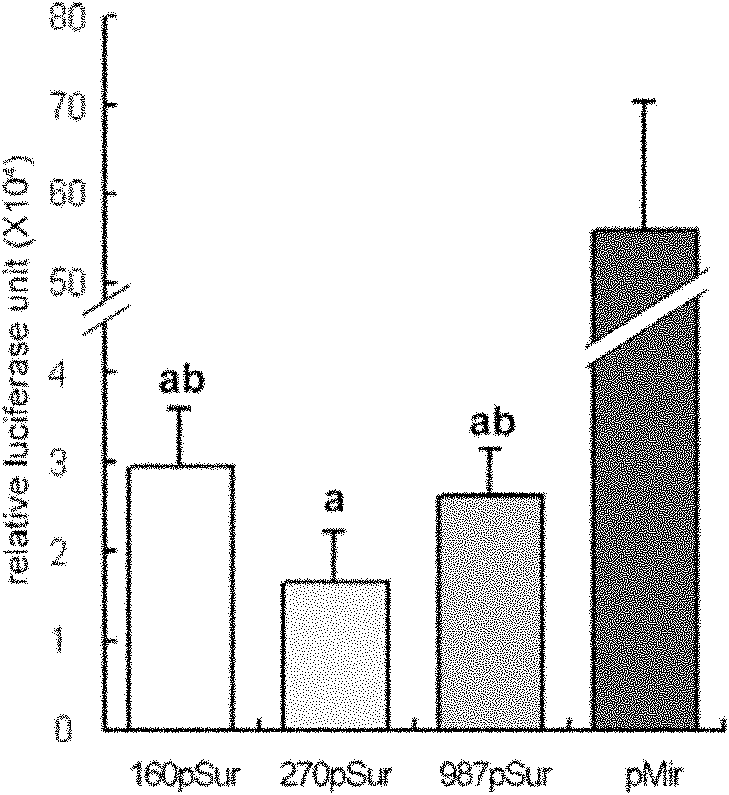
[0100] Example 2: Comparison of three kinds of promoter activation activities (antibody detection of target protein expression)
[0101] Materials: Three kinds of recombinant lentivirus supernatant Lenti-FG-161pSurAH, Lenti-FG-272pSurAH, Lenti-FG-990pSurAH obtained in Example 1, BIO-RAD electrophoresis instrument and electroporation instrument (BIO-RAD Company, USA), primary antibody Mouse anti-His antibody (Beyond Company), secondary antibody is horseradish peroxidase-labeled goat anti-mouse IgG (H+L) (Beyond Company), ECL luminescence kit (Beyond Company)
[0102] method:
[0103] 1. Introduce 293FT into a 24-well plate, and control the quantity to ensure that the cell density is about 70-80% the next day.
[0104] 2. Using Lip200 as a medium, transfect three plasmids, FG-161pSur-ApoH-pA, FG-272pSur-ApoH-pA and FG-990pSur-ApoH-pA, according to routine operations, and the amount of transfected plasmid per well is 800ng. Plasmid (ug): Lip2000 (ul) = 1: 1.5, and a negative co...
Embodiment 3
[0113] Example 3: Recombinant lentivirus Lenti-FG-272pSurAH infects Hela cells and 10T1 / 2 cells to induce cell death
[0114] Materials: Use the recombinant Lenti-FG-272pSurAH lentiviral supernatant obtained in Example 1, Olympus 1X71-FL fluorescence inverted microscope, Hela cells, 10T1 / 2 cells
[0115] Method: The recombinant lentiviral supernatant Lenti-FG-272pSurAH obtained in Example 1 was used to infect the tumor cell line Hela cells and the mesenchymal stem cell line 10T1 / 2 cells for 72 hours.
[0116] A. Fluorescence microscope observation
[0117] 1. The tumor cell line Hela cells and the mesenchymal stem cell line 10T1 / 2 cells were grown on coverslips for 24 hours.
[0118] 2. Infect tumor cells with the recombinant lentivirus supernatant Lenti-FG-272pSurAH obtained in Example 1, and continue culturing for 72 hours.
[0119] 3. The cells were washed twice with PBS.
[0120] 4. Add 5ul 7-AAD staining solution to 500ul Binding Buffer, mix well, and add to the above-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com