Engineering immune cell with suicide gene switch of targeting human mesothelin
An immune cell and suicide gene technology, applied in genetic engineering, blood/immune system cells, targeting specific cell fusion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
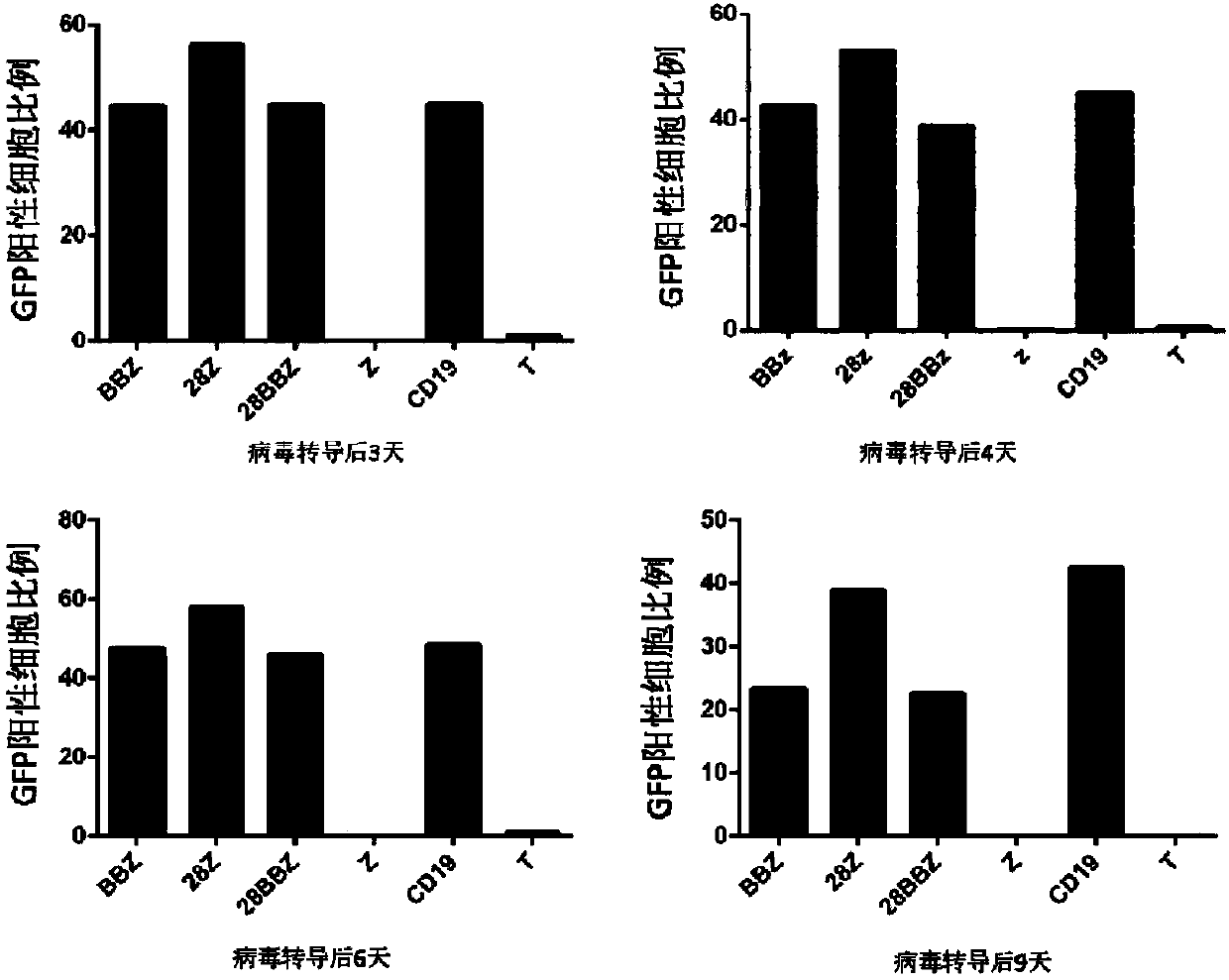
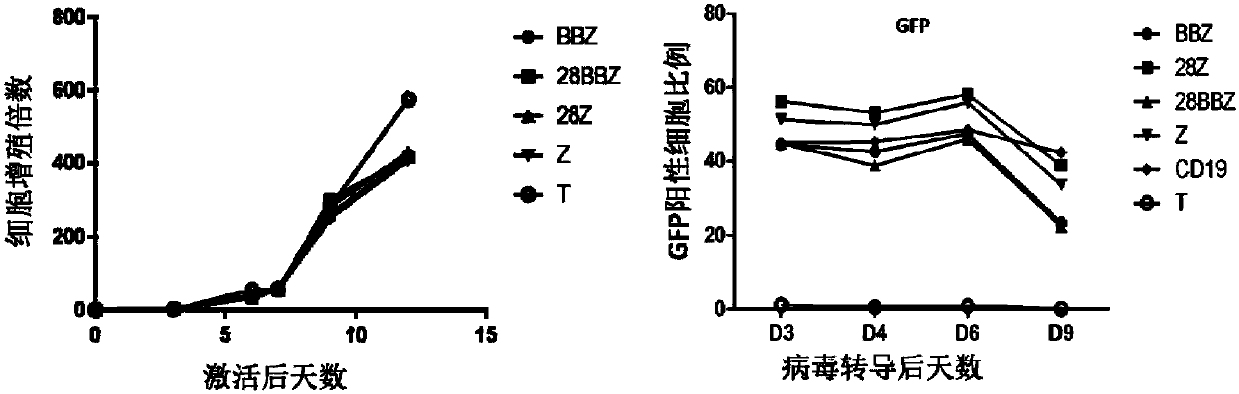
[0206] Isolation of PBMCs and Expansion of T Cells from Donor Blood
[0207] Mononuclear cells were isolated from umbilical cord blood, density gradient centrifugation was performed using Histopaque-1077 (Sigma-Aldrich), and T cells were enriched (EasySep human T cell enrichment kit, Stemcell Technologies), using conjugated anti-CD3 / anti-CD28 T cells were activated, cultured and expanded with magnetic beads; medium used was X-vivo15 (containing 5% FBS, 2mM L-glutamine, 1mM sodium pyruvate, 300IU / ml rhIL2); all cells were placed at 37°C, 5% CO 2 cultured in a constant temperature incubator.
Embodiment 2
[0209] Cell Culture and Construction
[0210] Cell lines expressing MSLN: OVCAR3 cells (human ovarian cancer cell line, HTB-161 TM ), HCT116 (human colon cancer cell line, CCL-247 TM ), CRL5826 (human lung cancer cells, CRL-5826, H226); K562 cells expressing MSLN / CD19 (human erythroleukemia cell line, -CCL-243), the above cells were cultured using RPMI 1640 medium; 293T (human kidney epithelial cell line cells, CRL-3216) were cultured using DMEM medium. All media were supplemented with 10% (v / v) fetal bovine serum and 100 U / ml penicillin and streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate
[0211] Among them, K562 cells expressing MSLN and CD19, and CRL5826, HCT116 and OVCAR3 expressing PDL-1 highly are stably transfected cell lines obtained by transferring MSLN, CD19 and PDL-1 antigens through lentiviral vectors, and then undergoing monoclonal screening. , can specifically express protein molecules such as MSLN, CD19 and PDL-1.
Embodiment 3
[0213] CAR structure design and transduction
[0214] meso-CAR structure, that is, the CAR structure targeting Mesothelin:
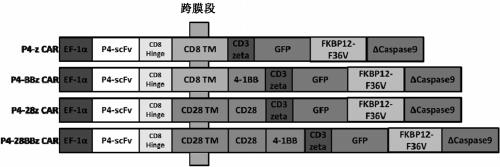
[0215] The method of the present invention constructs the first-generation, second-generation and third-generation CARs, all of which have an adjustable switch icasp9 (FKBP12-F36V-Caspase9) in their structures, connected through P2A, such as figure 1 shown.
[0216] The core structure of CAR includes the CD8 extracellular leader sequence, the scFv from P4 (specifically targeting mesothelin), the hinge from CD8 and the CD8 / CD28 transmembrane region. According to the presence or absence of co-stimulatory signals in intracellular segments, four meso-CARs were constructed, named CD3ζ, 4-1BB-CD3ζ, CD28-CD3ζ, and CD28-4-1BB-CD3. Named according to the different intracellular co-stimulatory regions, as shown in Table 1:
[0217] Table 1 Name of CAR-T cells
[0218] name
co-stimulatory signal
intracellular activation domain
P4-z-CAR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com