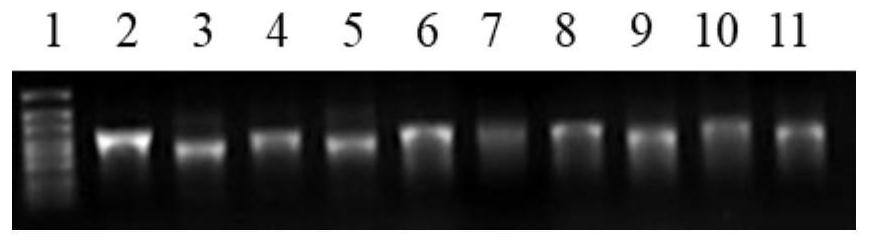
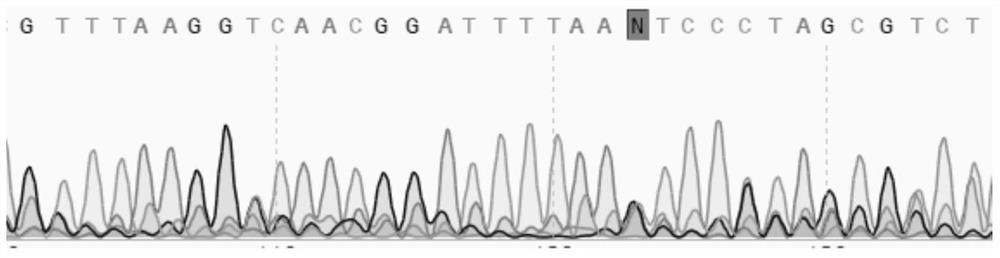
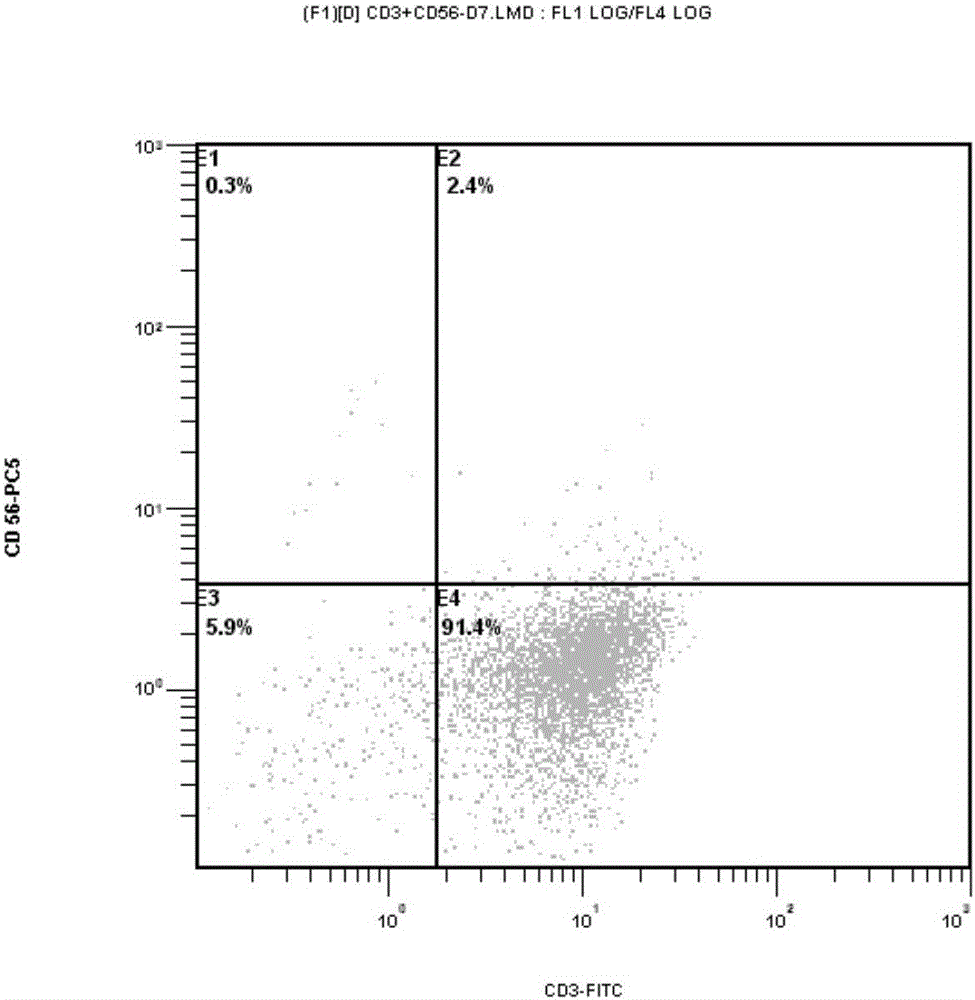
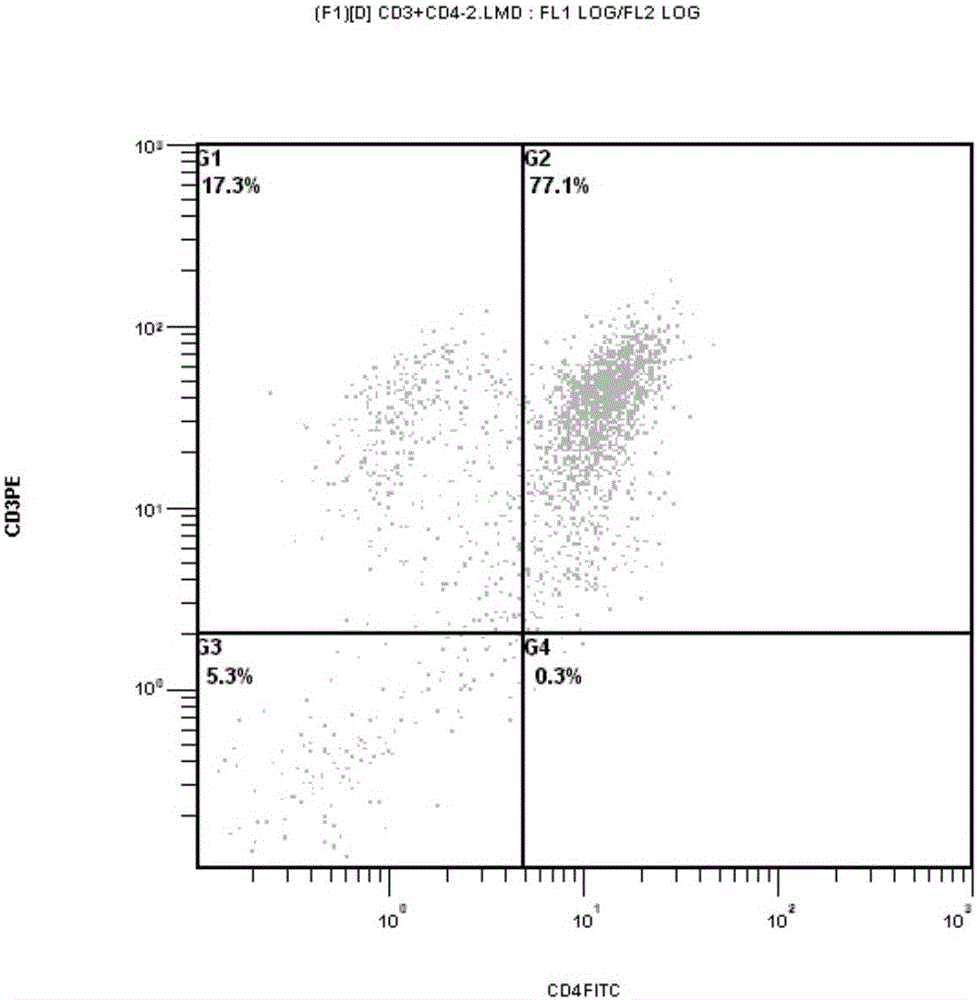
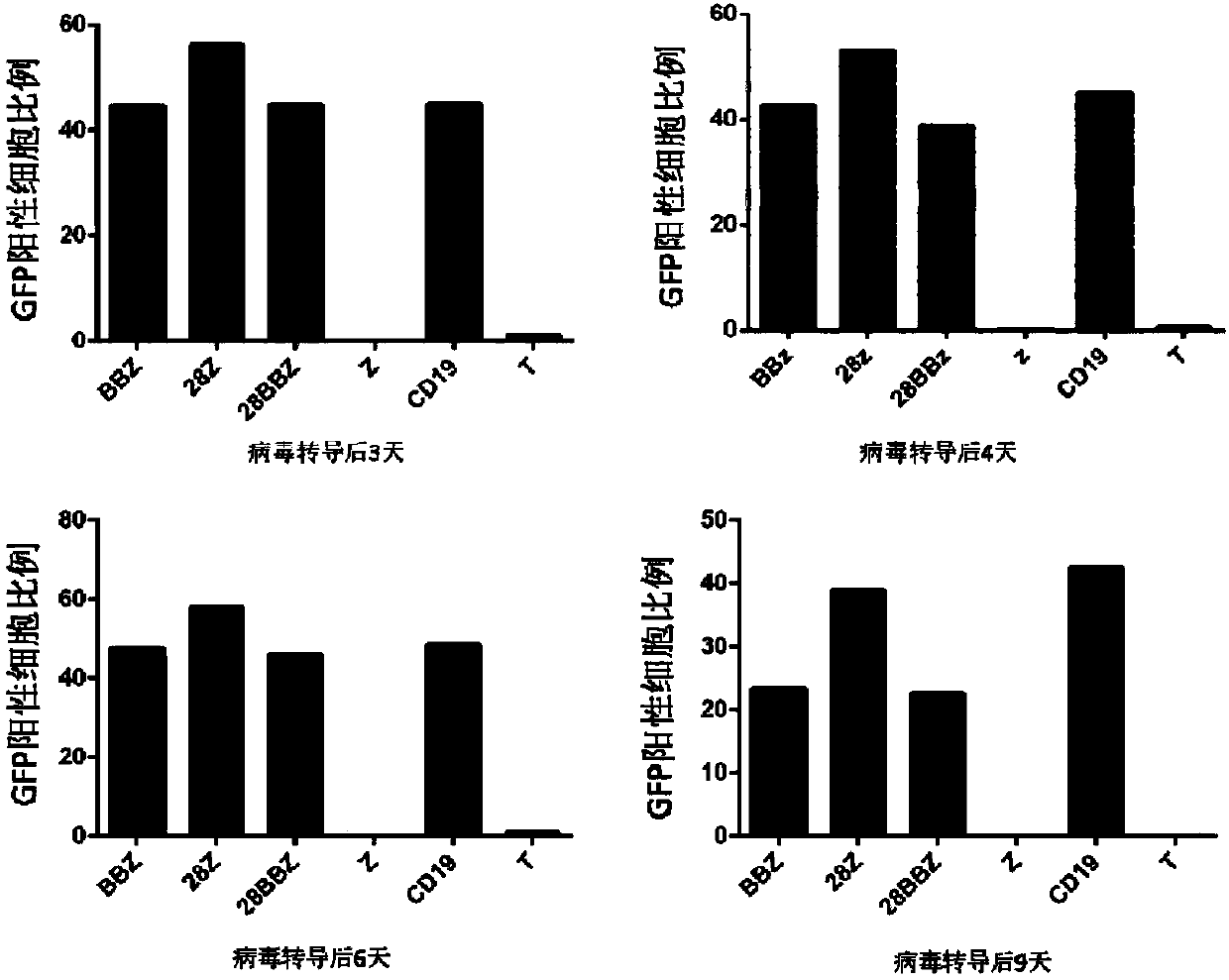
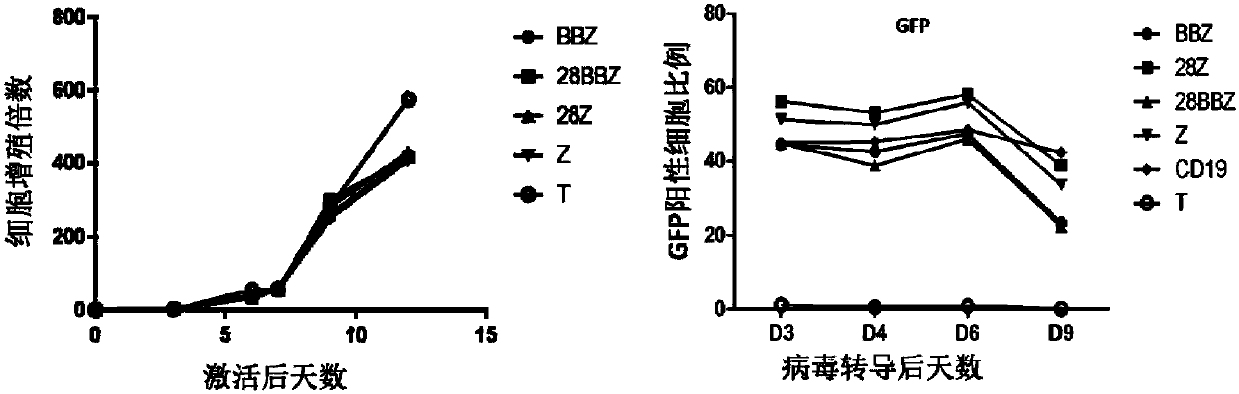
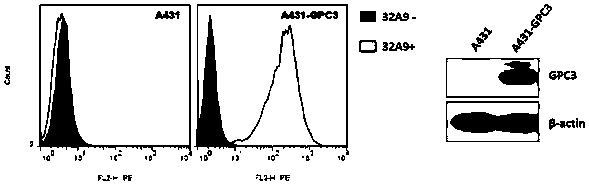
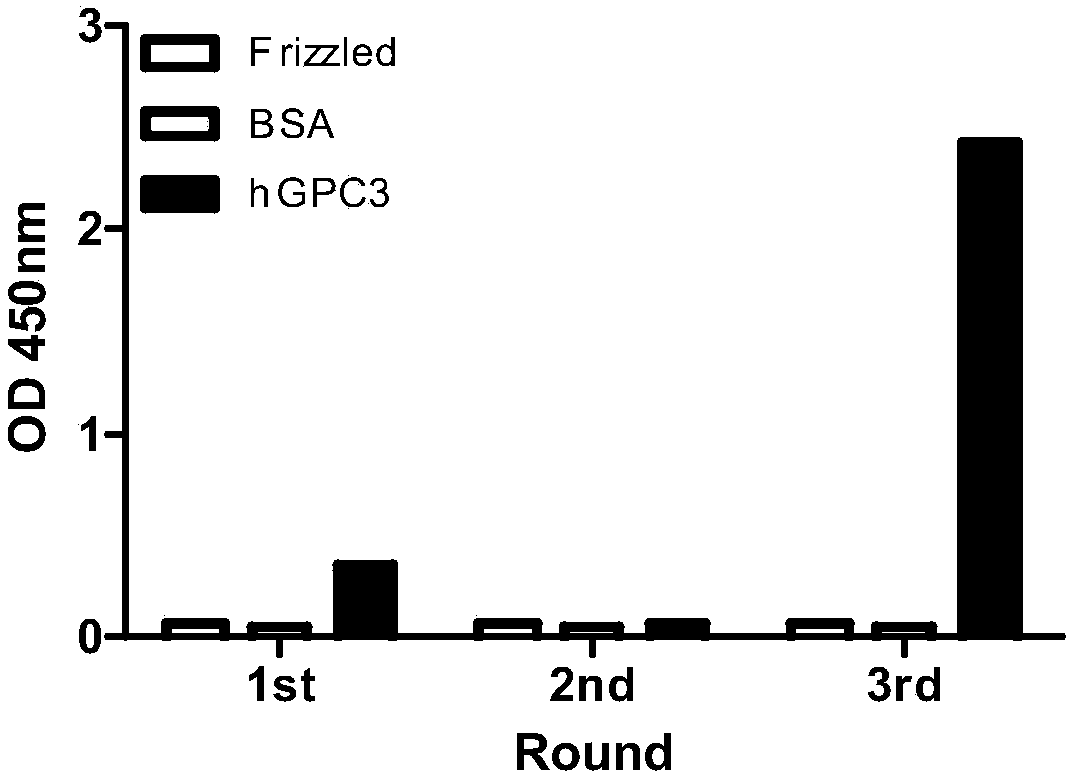
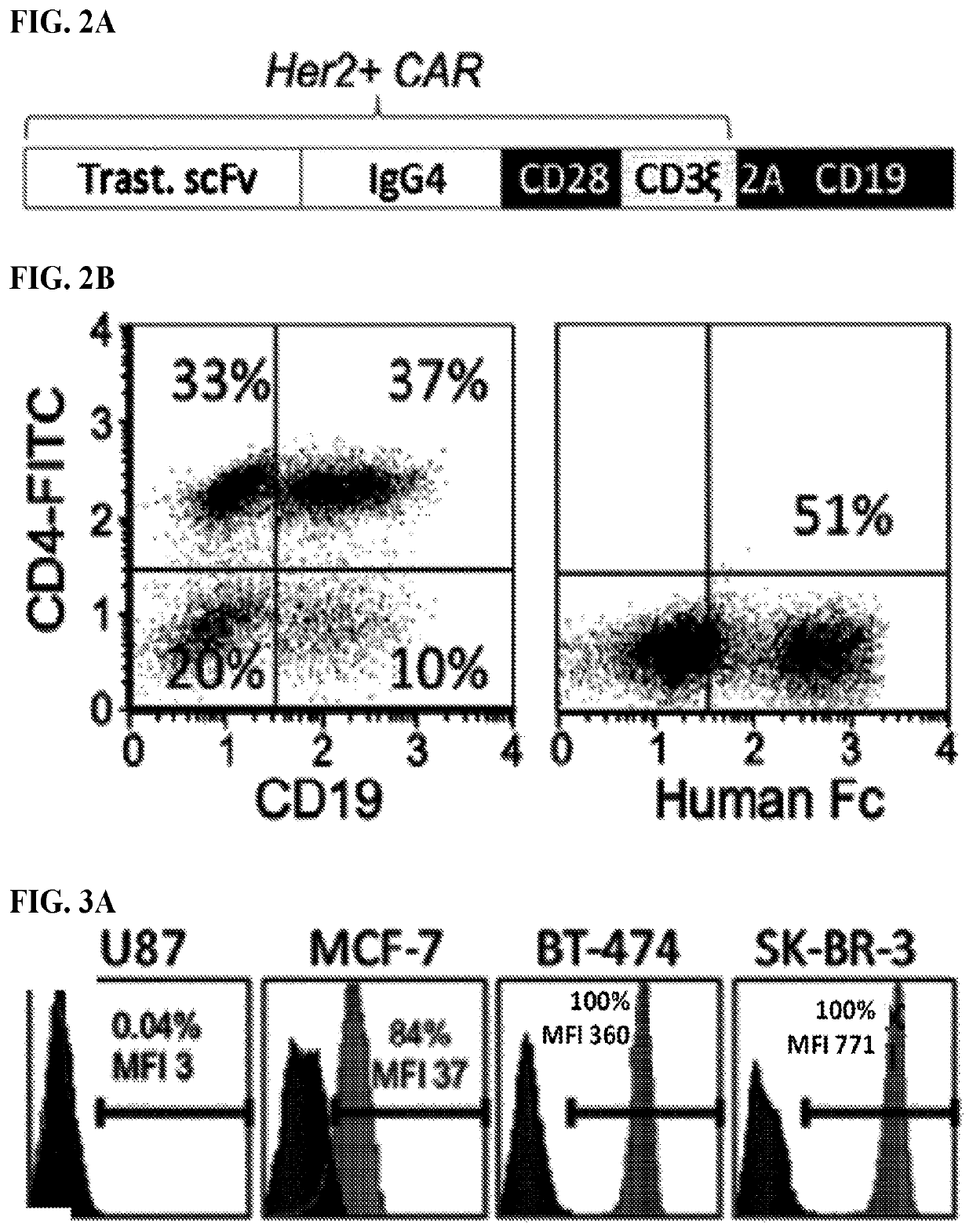
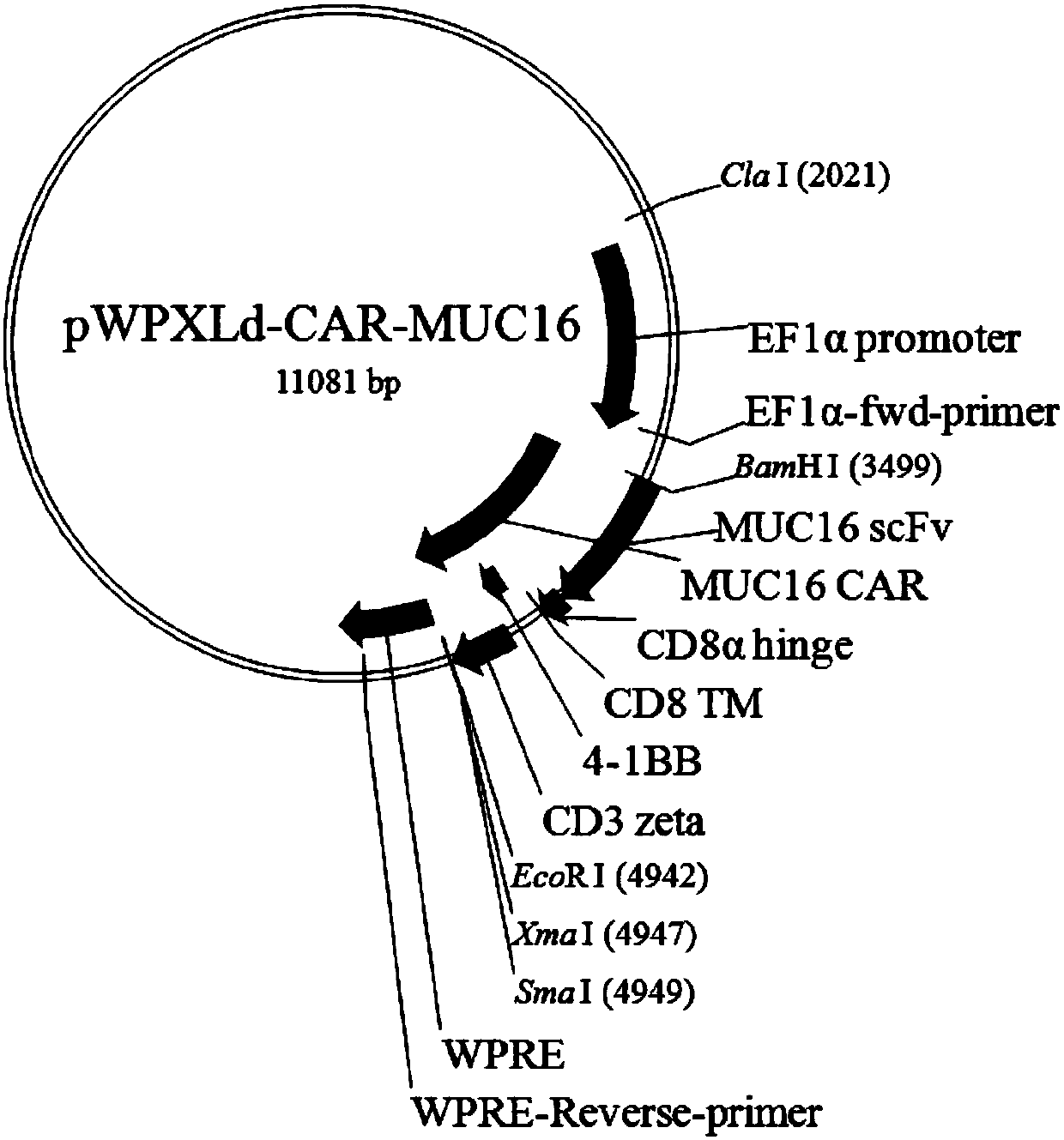
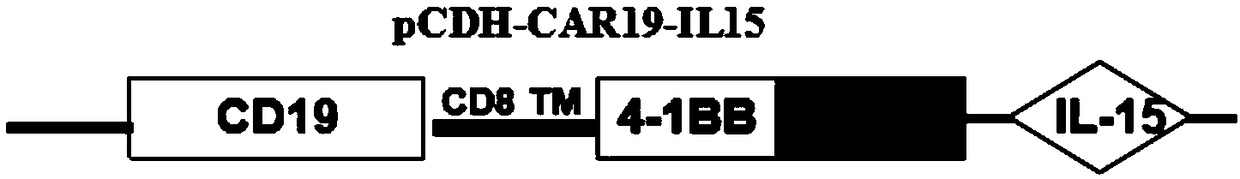
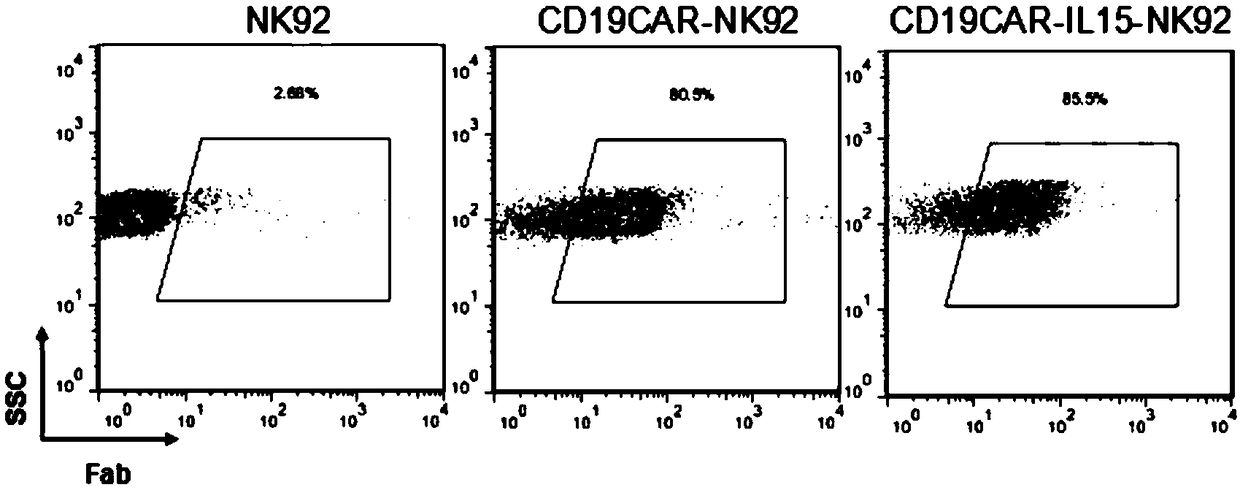
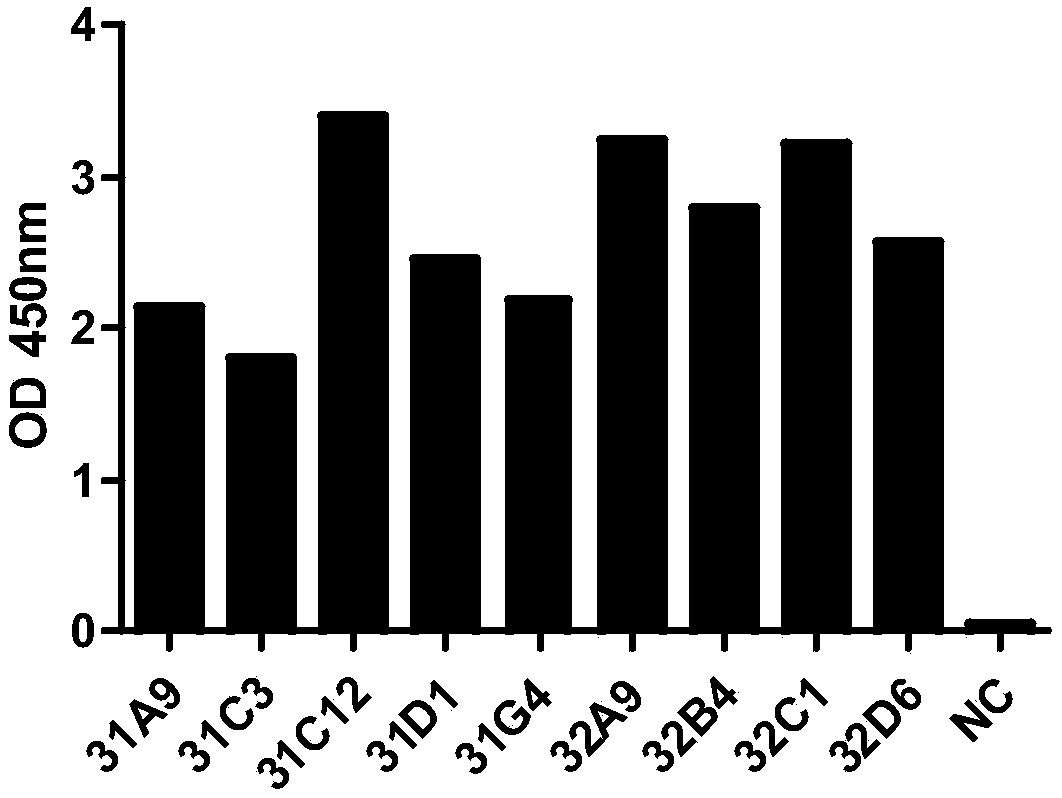Patents
Literature
40 results about "Chimeric Antigen Receptor T-Cell Therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
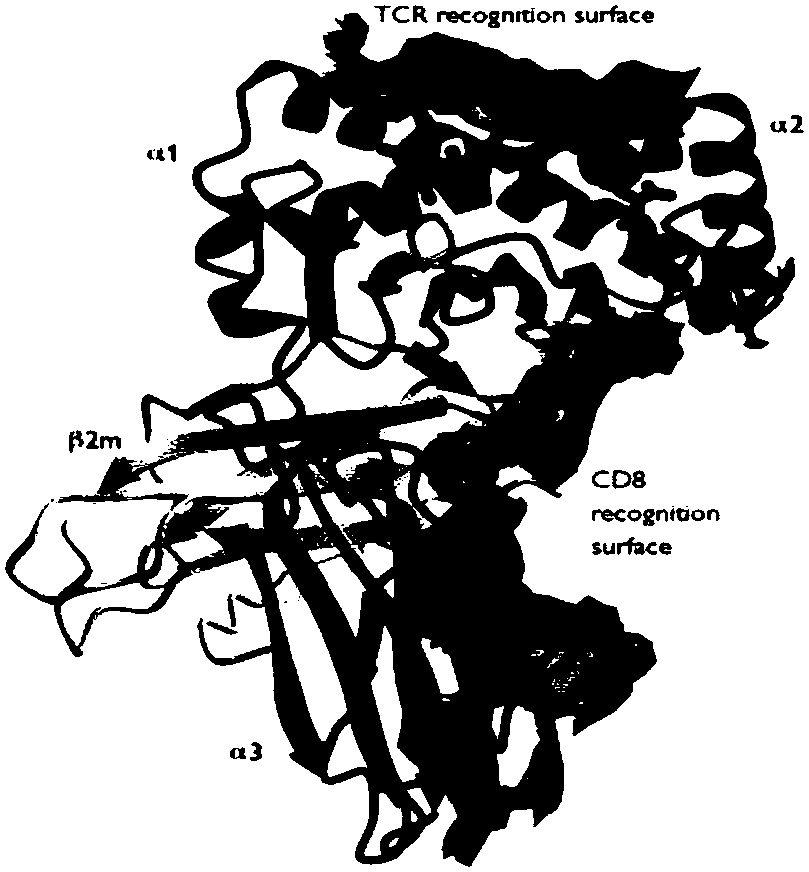
A type of treatment in which a patient's T cells (a type of immune system cell) are changed in the laboratory so they will attack cancer cells. T cells are taken from a patient's blood. Then the gene for a special receptor that binds to a certain protein on the patient's cancer cells is added in the laboratory. The special receptor is called a chimeric antigen receptor (CAR). Large numbers of the CAR T cells are grown in the laboratory and given to the patient by infusion. Chimeric antigen receptor T-cell therapy is being studied in the treatment of some types of cancer.
Chimeric antigen receptor dendritic cell (car-dc) for treatment of cancer
InactiveUS20170151281A1High proliferation rateIncrease productionPolypeptide with localisation/targeting motifPeptide/protein ingredientsIntracellular signallingDendritic cell
The current invention provides monocytic cells transfected with chimeric antigen receptor (CAR) to selectively home to tumors and upon homing differentiate into dendritic cells capable of activating immunity which is inhibitory to said tumor. In one embodiment of the invention, monocytic cells are transfected with a construct encoding an antigen binding domain, a transcellular or structural domain, and an intracellular signaling domain. In one specific aspect of the invention, the antigen binding domain interacts with sufficient affinity to a tumor antigen, capable of triggering said intracellular domain to induce an activation signal to induce monocyte differentiation into DC.
Owner:MYELOID THERAPEUTICS INC
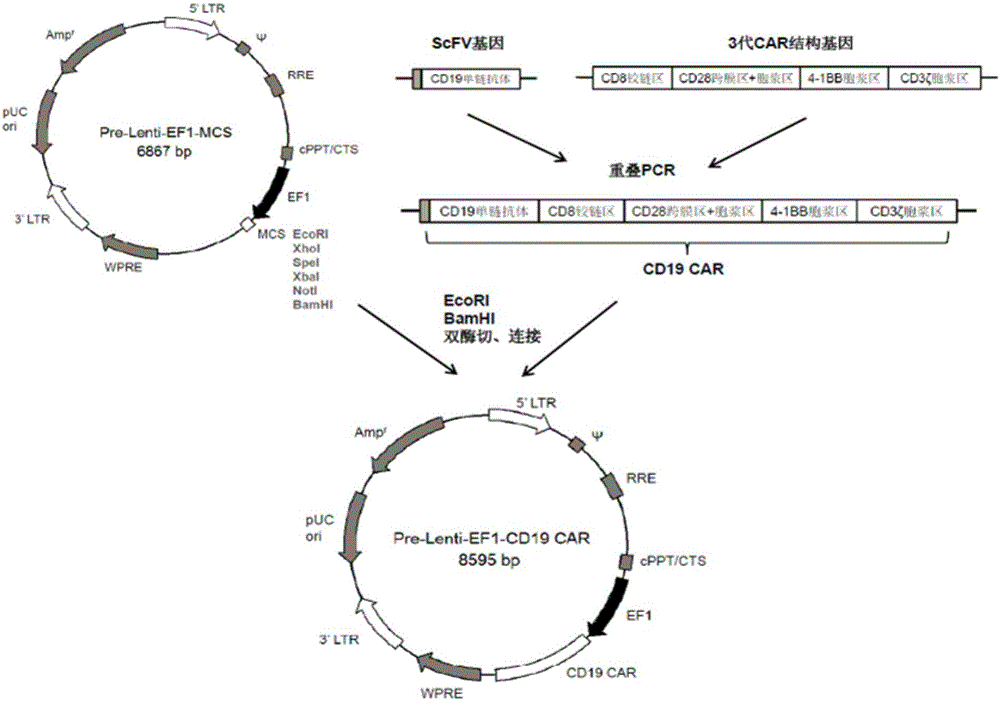
CD19 targeted CAR (chimeric antigen receptor)-T cell, preparation method and application
InactiveCN107287164AIncrease lethalityHigh transduction efficiencyGenetically modified cellsMammal material medical ingredientsCAR T-cell therapySingle-Chain Antibodies
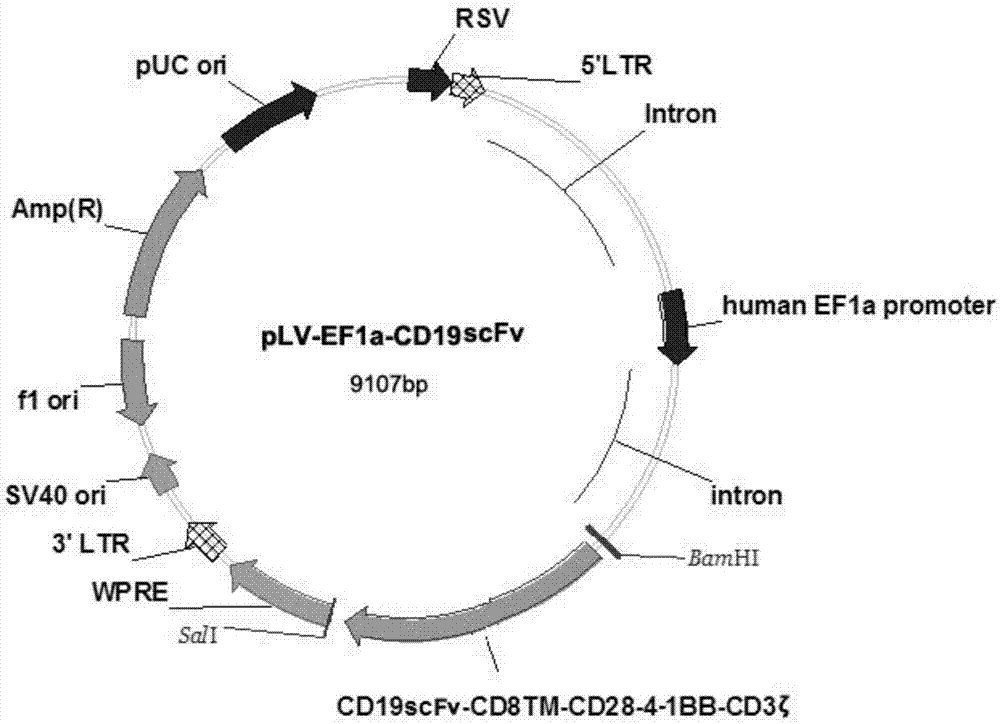
The invention provides a CD19 targeted CAR (chimeric antigen receptor)-T cell, a preparation method and an application and relates to the field of immune cells. The activation capacity of T cells in CAR-T cell therapy can be improved, the problem of insufficient transfection efficiency can be solved, and the CAR-T cell has a high killing capacity for CD19. The CD19 targeted CAR-T cell expresses a CD19 targeted CAR gene on surface, and the CD19 targeted CAR gene is formed by connecting a single-chain antibody CD19ScFv of CD19, a hinge region and a transmembrane region of CD8, an intracellular signal structure of CD28, an intracellular signal structure of 4-1BB and an intracellular signal structural domain of CD3zeta in series, and the base sequence of the CD19 targeted CAR gene is shown as SEQ ID NO:2.
Owner:青岛见康华美医学检验有限公司
Recombinant nucleic acid molecule for transcribing circular RNA and application of recombinant nucleic acid molecule in protein expression
ActiveCN112481289AImprove expression levelImprove expression efficiencyTumor rejection antigen precursorsSsRNA viruses positive-senseDendritic cellTGE VACCINE
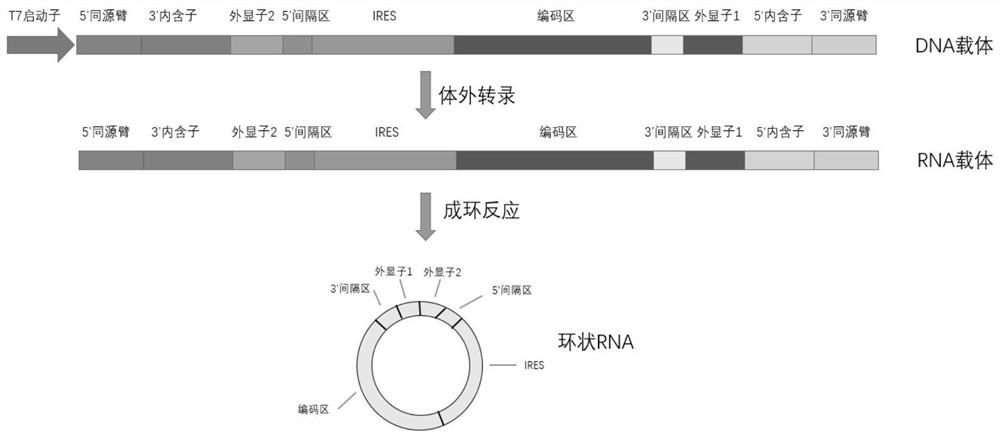
The invention relates to a recombinant nucleic acid molecule for transcribing circular RNA and application of the recombinant nucleic acid molecule in protein expression. Specifically, the invention relates to the recombinant nucleic acid molecule for transcribing circular RNA, a recombinant expression vector, linear RNA, circular RNA, a recombinant host cell, a pharmaceutical composition and a method for preparing protein. The recombinant nucleic acid molecule is transcribed to form circular RNA containing a specific IRES element, the IRES element can improve the protein expression level of the circular RNA in eukaryotic cells, efficient and durable expression of protein is achieved, and the recombinant nucleic acid molecule has important application values for preparing mRNA infectious disease vaccines, therapeutic mRNA tumor vaccines and mRNA-based dendritic cell tumor vaccines, and in the fields of gene therapy based on mRNA, chimeric antigen receptor T cell therapy based on mRNA,protein supplement therapy and the like.
Owner:SUZHOU CUREMED BIOMEDICAL TECH CO LTD
Targeting chimeric antigen receptor modified immune cell as well as preparation method and application thereof
InactiveCN105906720ABroaden the range of targetsAccurate targetAntibody mimetics/scaffoldsMammal material medical ingredientsSequence signalHinge region
Owner:WUHAN HAMILTON BIOTECH
Chimeric antigen receptor immune cell provided with safety switch as well as preparation method and application of chimeric antigen receptor immune cell
InactiveCN106755023AImprove effectivenessImprove securityGenetic material ingredientsMammal material medical ingredientsAbnormal tissue growthAntigen receptors
The invention relates to a chimeric antigen receptor (CAR) immune cell provided with a safety switch as well as a preparation method and an application of the CAR immune cell. The CAR immune cell carrying the safety mechanism (the safety switch) comprises a CAR coding nucleotide sequence, wherein the structure of the nucleotide sequence comprises a receptor structural domain for recognizing tumor-specific antigen or tumor-associated antigen, a transmembrane-stimulation structural domain, a CD3[zeta] stimulating signal transduction region and a suicide gene region. The CAR immune cell can be obtained as different immunological effect cells are amplified and a CAR sequence carrying a suicide mechanism is transduced; corresponding antigens of tumor cells are recognized by virtue of CAR; and the CAR immune cell can generate a specific killing effect on the tumor cells. The CAR immune cell, when used, is infused in a gradient mode and dynamic change in related cell factor levels is monitored; in case of need, a suicide gene can be started by virtue of drugs to scavenge the immunological effect cells, so that optimal balance between safety and a curative effect is achieved; therefore, the safety of the technology applied to the treatment of tumors in the clinical field is guaranteed to the greatest extent.
Owner:AFFILIATED HOSPITAL CHINA ACADEMY OF MILITARY MEDICAL SCI +1
CAR-T (chimeric antigen receptor T cell) for targeting CD19 and application of CAR-T
ActiveCN107827991ARestrict growthPrevent proliferationMammal material medical ingredientsImmunoglobulinsSequence signalSingle-Chain Antibodies
The invention discloses a CAR-T (chimeric antigen receptor T cell) for targeting CD19 and an application of the CAR-T. A CAR for preparing the CAR-T comprises interleukin 2 signal peptides, an anti-CD19 single-chain antibody, a CD8 protein molecule hinge region, a transmembrane region, an intracellular signal structure region and a CD3 zeta protein molecule intracellular signal conduction structure region which are connected in series sequentially and has the amino acid sequence shown in SEQ ID NO:9. The CAR-T is applied to preparation of a medicine or a preparation for treating hematologicalmalignancy, wherein the hematological malignancy comprises CD19-positive B-cell acute lymphocytic leukemia, diffuse large B cell lymphoma and non-hodgkin's lymphoma.
Owner:英普乐孚生物技术(上海)有限公司
Engineering immune cell with suicide gene switch of targeting human mesothelin
ActiveCN109593721APolypeptide with localisation/targeting motifImmunoglobulin superfamilyDiseaseAbnormal tissue growth
The invention provides an engineering immune cell with a suicide gene switch of targeting human mesothelin (MSLN). Particularly, the invention provides a chimeric antigen receptor T cell of the targeting human mesothelin, a CAR (Chimeric Antigen Receptor) structure of the cell comprises a cell suicide element, and the expression of a PD1 gene in the cell is silent. Particularly, the CAR structurecomprises a CAR basic structure and the cell suicide element at the same time, the CAR basic structure and the cell suicide element are independent of each other, and the corresponding functions of the CAR basic structure and the cell suicide element are non-interfering. In addition, the silent expression of the PD1 gene in the cell has a synergistic effect with the CAR structure, the tumor killing effect is strengthened, and the disease does not relapse easily.
Owner:GRACELL BIOTECH SHANGHAI CO LTD
Chimeric antigen receptor based on BMCA nano-antibody sequence and application thereof
PendingCN109694413AStrong specificityImprove tumor killing abilityPolypeptide with localisation/targeting motifNGF/TNF-superfamilyMalignant lymphomaAntigen Binding Fragment
The invention relates to the technical field of cellular immunity, and discloses a chimeric antigen receptor based on a BMCA nano-antibody sequence and an application thereof, wherein the chimeric antigen receptor includes an extracellular structural domain, a transmembrane structural domain and a primary signal transduction domain. The extracellular structural domain includes an alpaca anti-BCMAantibody or an antigen binding fragment of an alpaca BCMA polypeptide or multiple epitopes. The chimeric antigen receptor is mainly applied in tumor treatment drugs, tumors are blood-related tumor diseases, and the blood-related tumor diseases mainly include various types of leukemia and malignant lymphoma. According to the chimeric antigen receptor based on the BMCA nano-antibody sequence and theapplication thereof, the expression sequence is shorter, and the limited space of a lentiviral vector is saved. The chimeric antigen receptor T cell constructed based on the antibody has better specificity and stronger tumor killing ability.
Owner:深圳市前海精准生物科技有限公司
Anti-GPC3 fully-humanized antibody, and chimeric antigen receptor cell and application thereof
ActiveCN109021108AStrong specificityHigh affinityGenetically modified cellsMammal material medical ingredientsHeavy chainBacteriophage
The invention relates to an anti-GPC3 fully-humanized antibody, and a chimeric antigen receptor cell and application thereof. The antibody at least has one selected from a group consisting of heavy chain CDR1, heavy chain CDR2, heavy chain CDR3, light chain CDR1, light chain CDR2 and light chain CDR3. The antibody can be used for preparing a cell or conjugate, a diagnostic kit and a drug or pharmaceutical composition; a nucleic acid in the invention is used for preparing the anti-GPC3 fully-humanized antibody, the drug or the pharmaceutical composition; the cell or conjugate is used for preparing the drug or the pharmaceutical composition; the drug or the pharmaceutical composition has anti-tumor effect against a GPC3-positive tumor. According to the invention, the anti-GPC3 fully-humanized antibody is screened out by using a phage display technology, and the screened antibody has good specificity and high affinity; and cells using the antibody, e.g., chimeric antigen receptor T-cells,have effective killing effect on GPC3-positive tumors.
Owner:NANJING MEDICAL UNIV
Chimeric antigen receptor T cells as well as preparation method and application thereof
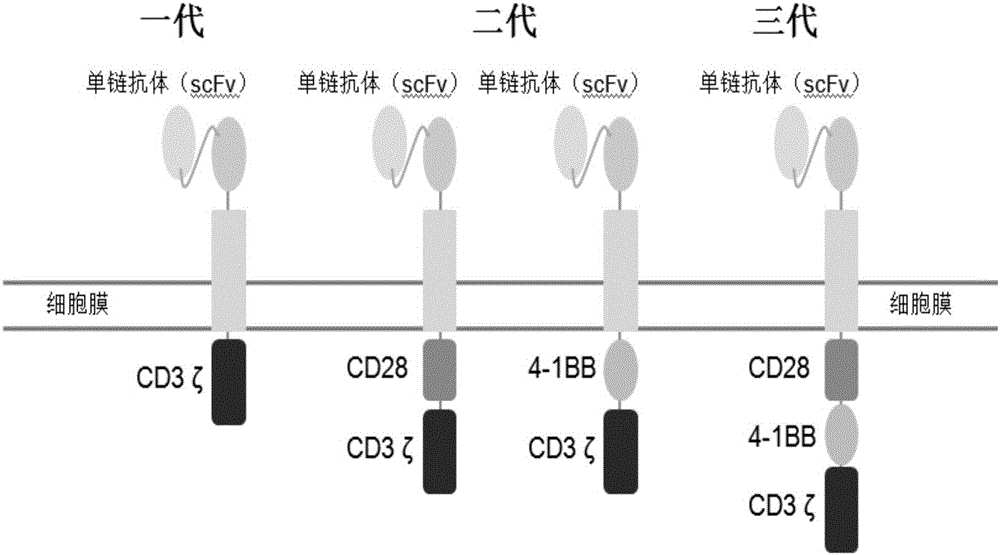
InactiveCN106754725AEfficient killingGenetically modified cellsMammal material medical ingredientsT cellChimeric Antigen Receptor T-Cell Therapy
The invention discloses chimeric antigen receptor T cells as well as a preparation method and an application thereof. The surfaces of the T cells express chimeric antigen receptor CD19 CAR genes, with the base sequence shown as SEQ ID NO: 3. The invention further discloses a preparation method and an application of the chimeric antigen receptor T cells. Two kinds of co-stimulatory signals are added into the structures of third-generation CD19 CAR vectors to construct lentiviral vectors, the effects of the third-generation CD19 CART are observed in cell and animal experiments, and the third-generation CD19 CART is proved to have the capability of effectively killing tumor cells.
Owner:SHENZHEN PREGENE BIOPHARMA CO LTD
High-affinity anti-MSLN antibody and applications thereof
PendingCN107840891AHigh affinityImprove securityImmunoglobulins against cell receptors/antigens/surface-determinantsNucleic acid vectorSingle-Chain AntibodiesAntiendomysial antibodies
The present invention provides a high-affinity anti-MSLN antibody and applications thereof, and specifically relates to an antibody having high affinity to MSLN, wherein the antibody is a single-chainantibody, has a heavy chain variable region and a light chain variable region, can be used for anti-MSLN-based chimeric antigen receptor T cell therapy methods, and has significant antitumor effects.
Owner:SHANGHAI GENBASE BIOTECH CO LTD
Universal chimeric antigen receptor T cell preparation technology
InactiveCN109456943AHydrolasesStable introduction of DNAHLA-BChimeric Antigen Receptor T-Cell Therapy
The invention relates to a universal chimeric antigen receptor T cell, a preparation method thereof and an application of the cell, and particularly provides a CAR (chimeric antigen receptor)-T cell.Binding of the CAR and cells HLA-A / HLA-B and TCR is inhibited, and TCR (T cell receptor) gene expression of the cell is silenced. The universal CAR-T cell can be used for treating allogenic tumors, GVHD (graft-versus-host disease) and HVG (host versus graft) reaction are avoided in allogenic infusion, and survival and anti-tumor effects of the allogenic CAR-T cell in a receptor are improved.
Owner:GRACELL BIOTECH SHANGHAI CO LTD
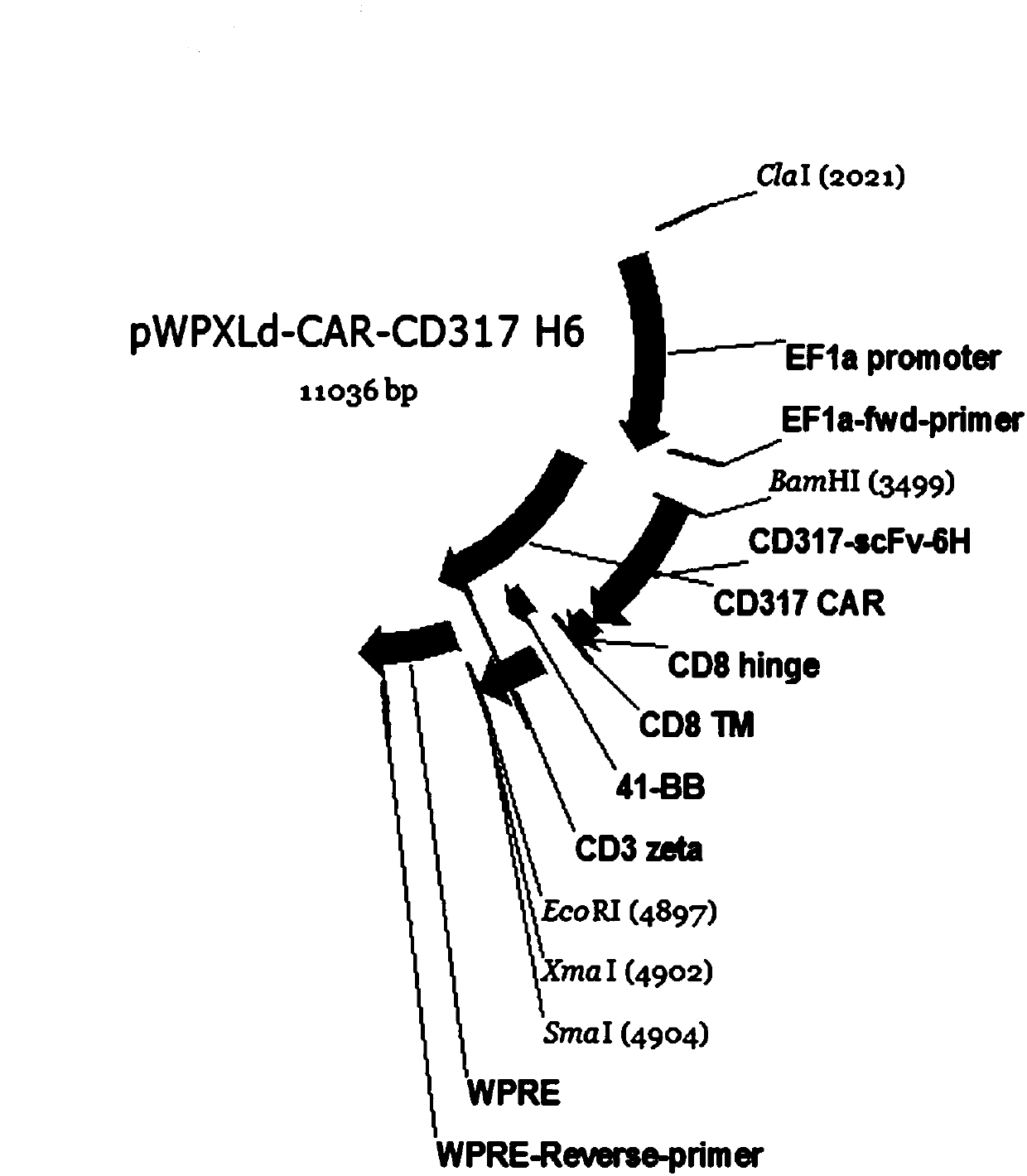
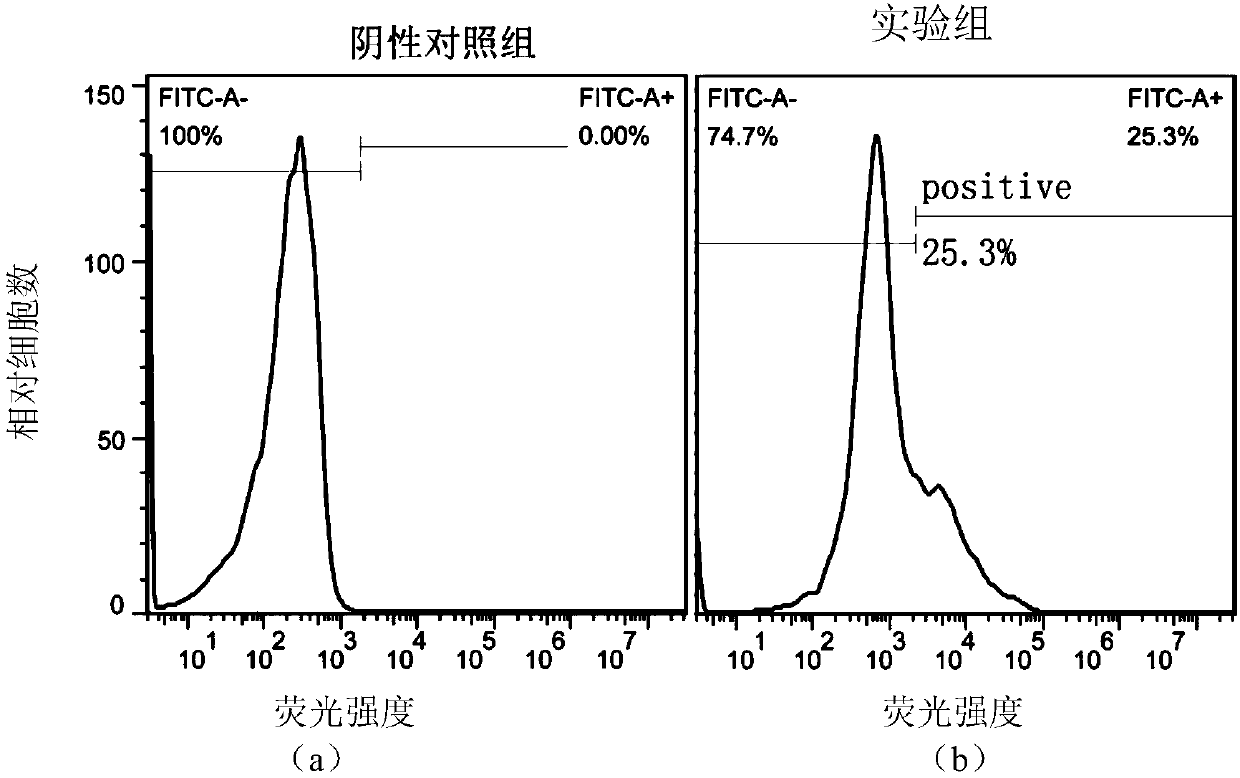
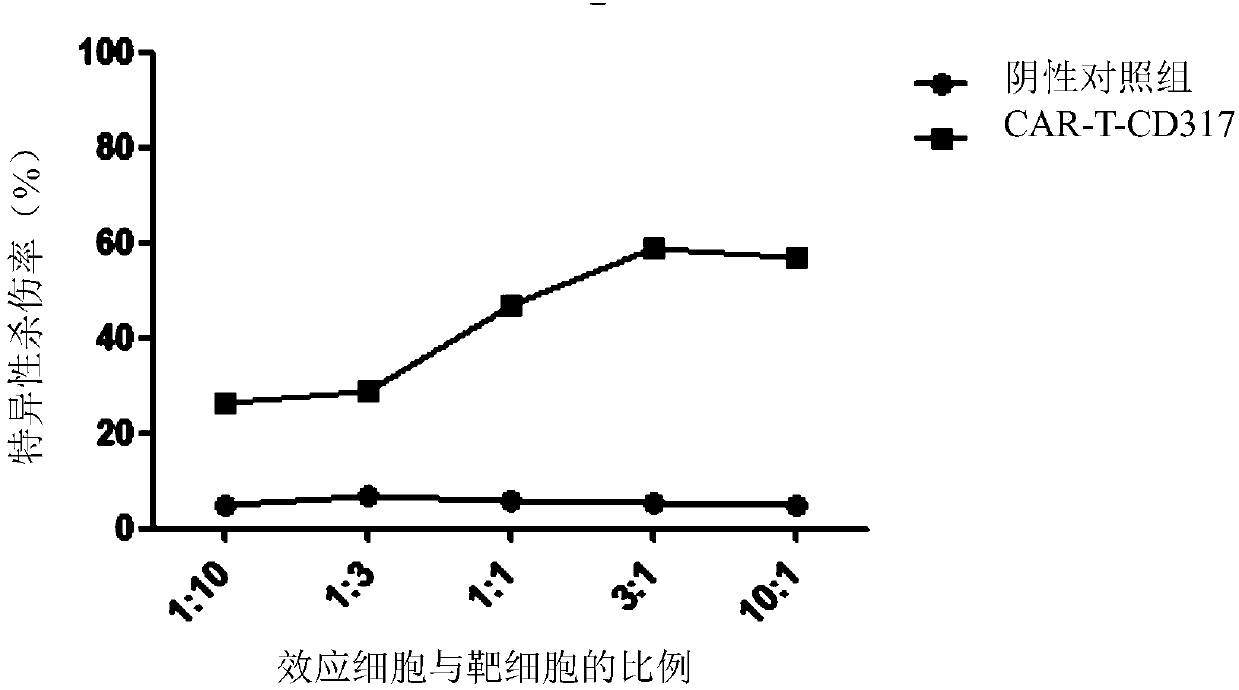
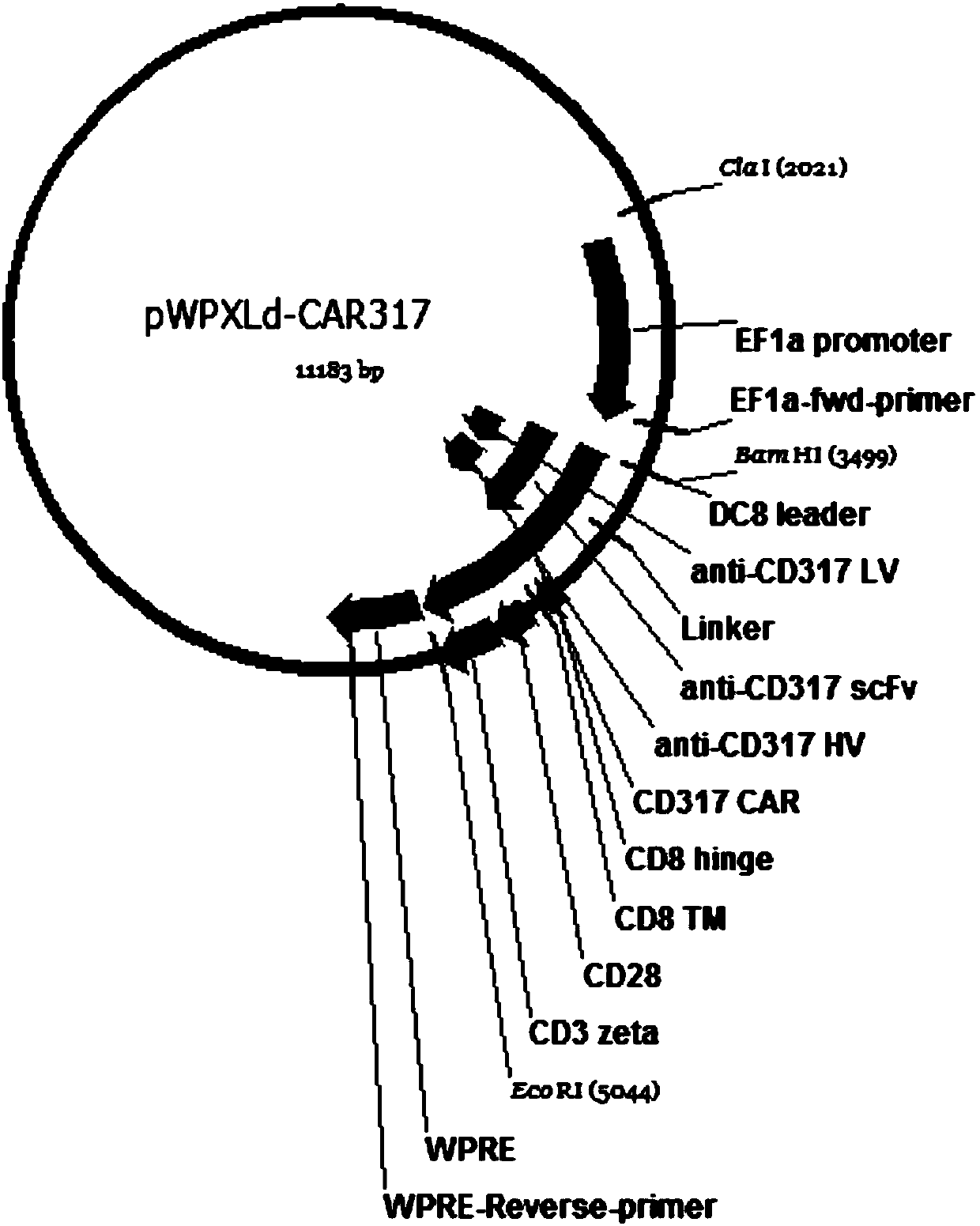
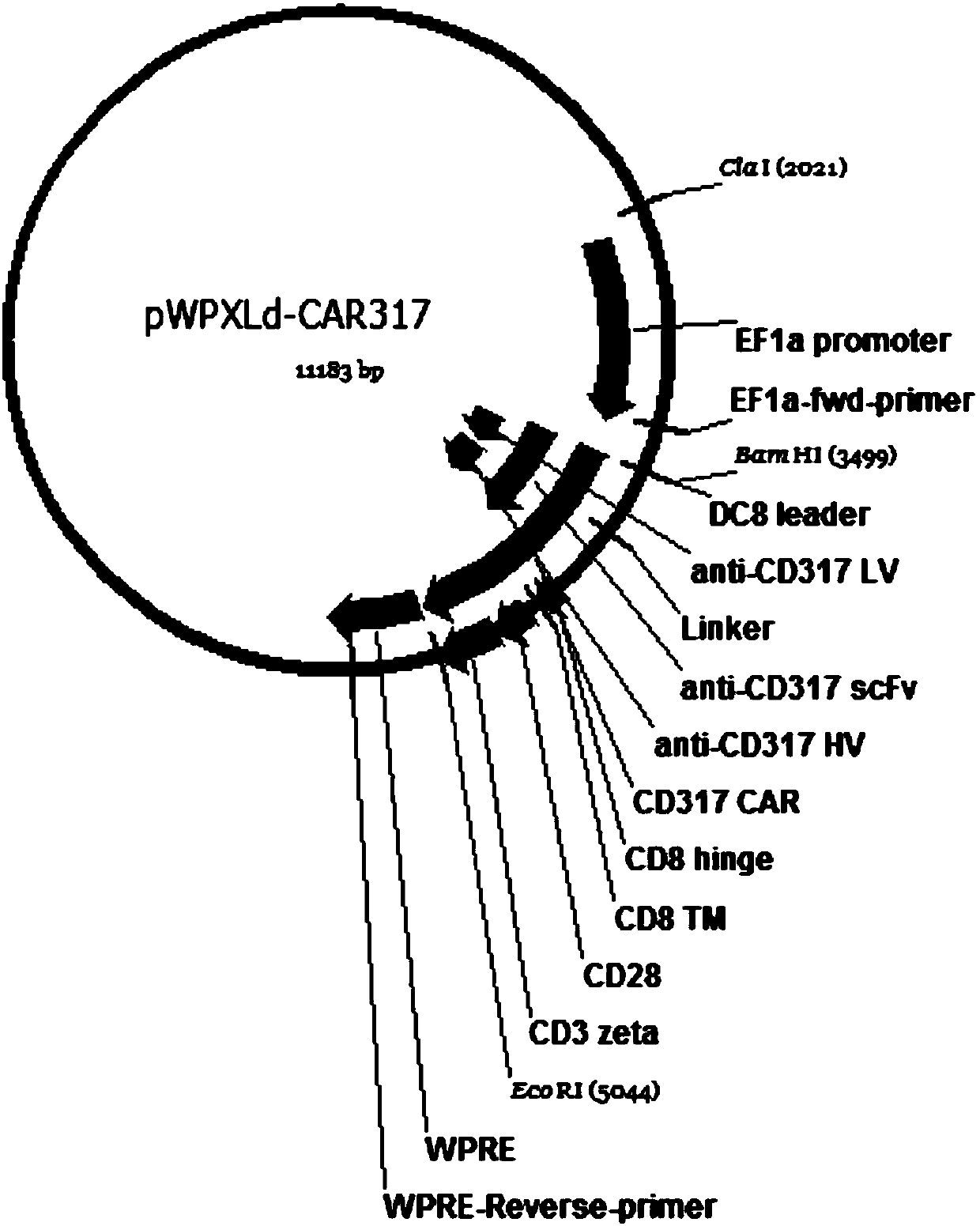
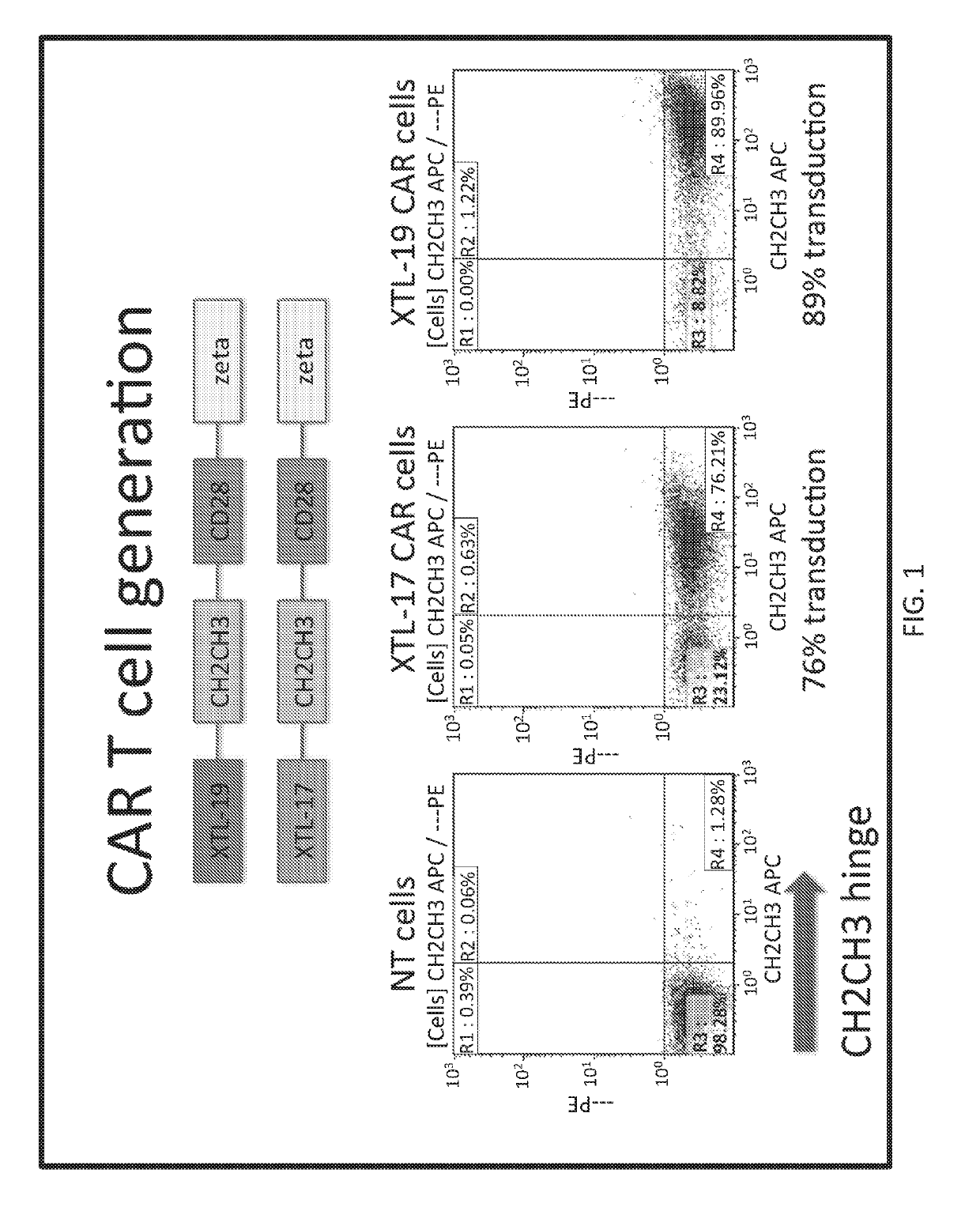
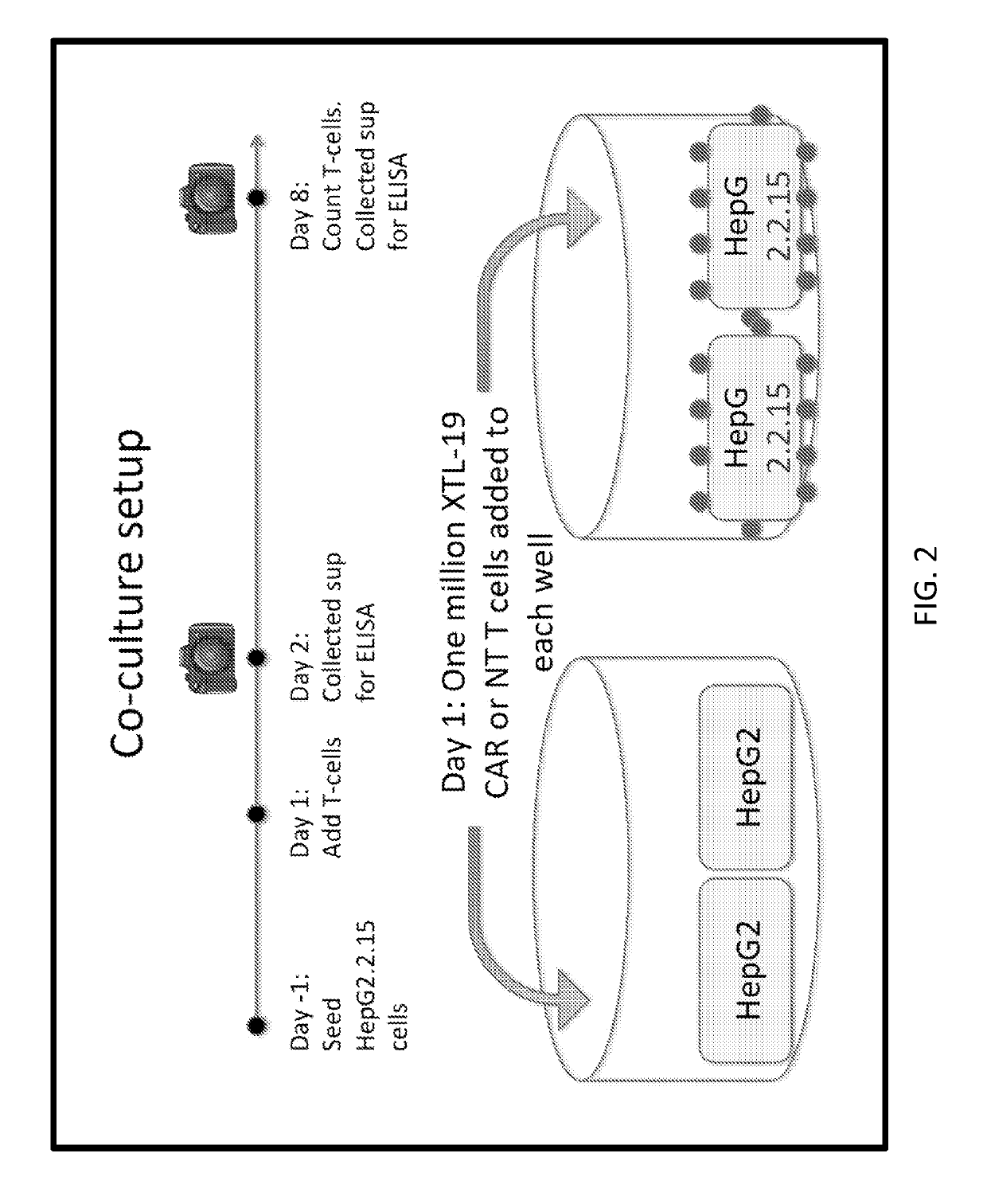
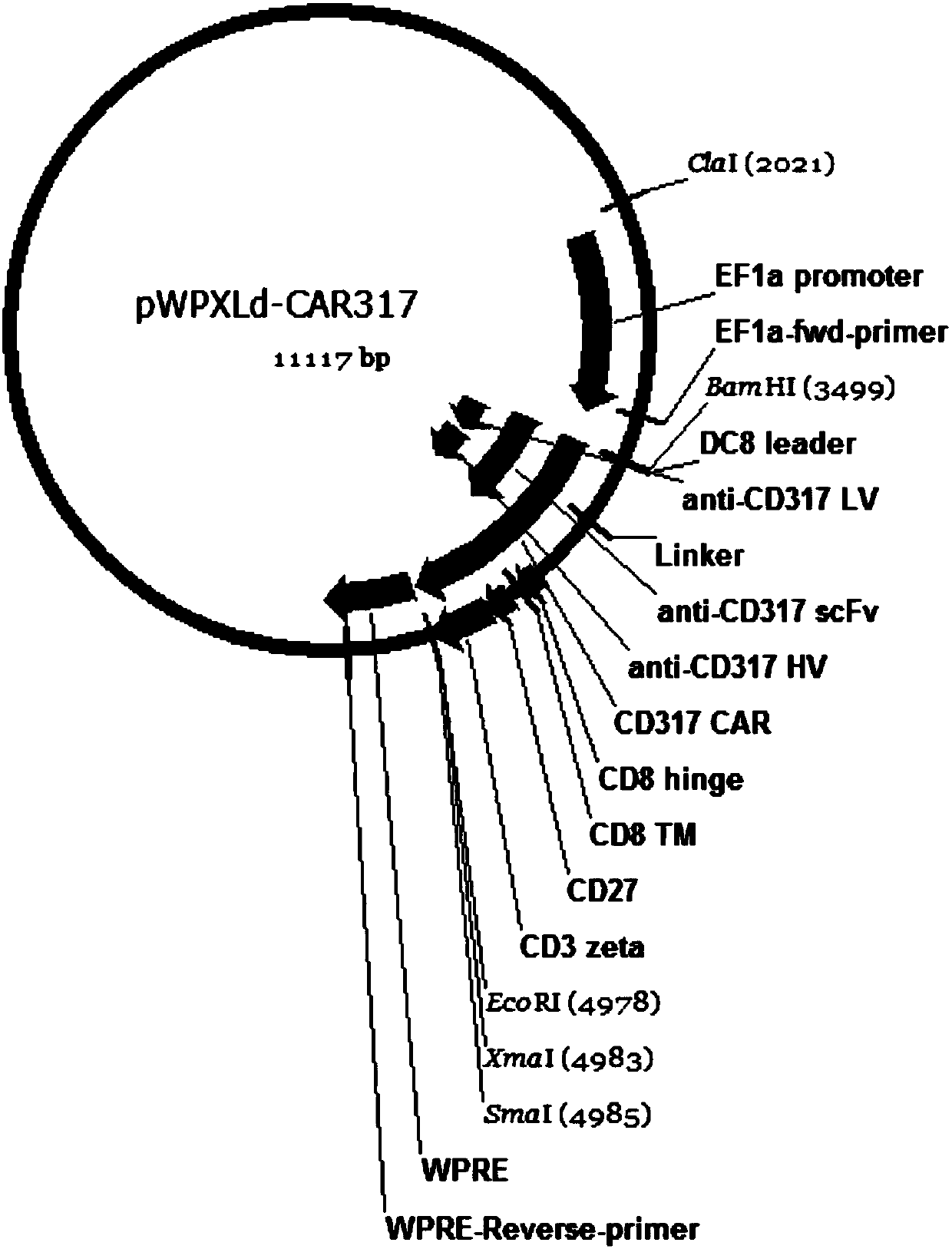
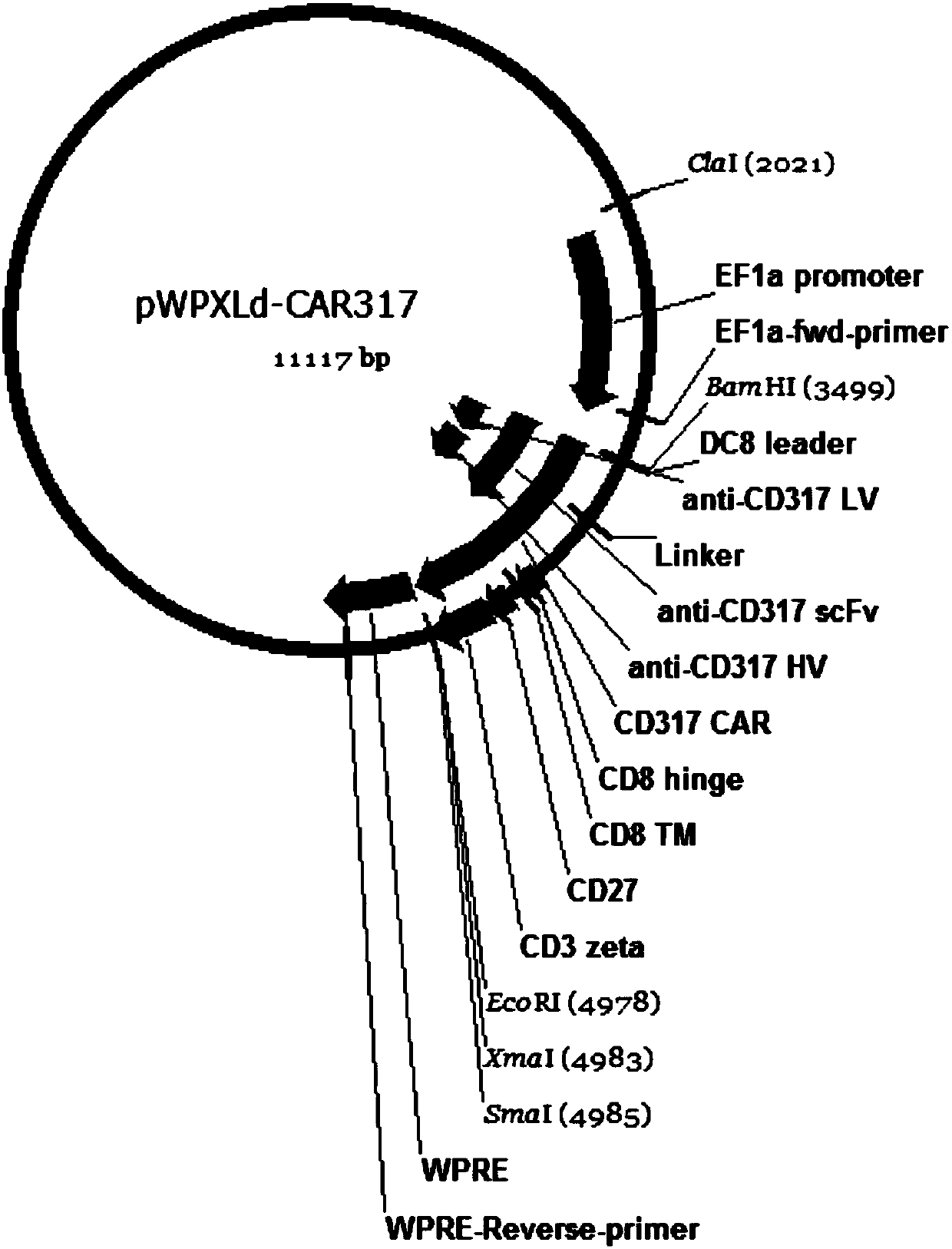
Single-chain antibody targeting CD317, chimeric antigen receptor T cell, preparation method and application thereof
InactiveCN109836496AImprove targetingPromote amplificationImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationAntigenSingle-Chain Antibodies
The present invention provides a single-chain antibody targeting CD317, the single-chain antibody targeting CD317 comprises an amino acid sequence shown in SEQ ID NO: 1, and the single-chain antibodytargeting CD317 is a humanized single-chain antibody. The present invention also provides a chimeric antigen receptor T cell comprising the single-chain antibody targeting CD317, wherein the chimericantigen receptor targeting CD317 can specifically target CD317, the expansion of T cells in patients is promoted, the tumor cells can be efficiently and specifically killed, and damage is hardly generated on the normal cells. In addition, the chimeric antigen receptor T cell targeting CD317 produced by humanized single-chain antibody can keep the ability of self-renewal and tumour lethality. The invention also provides a preparation method and an application of the chimeric antigen receptor T cell targeting CD317.
Owner:SHENZHEN BINDEBIOTECH CO LTD
Combination Therapies With Recombinant Listeria Strains
InactiveUS20180153974A1Enhanced anti-tumor T cell responseAntibody mimetics/scaffoldsFusion with degradation motifTransfer cellGenus Listeria
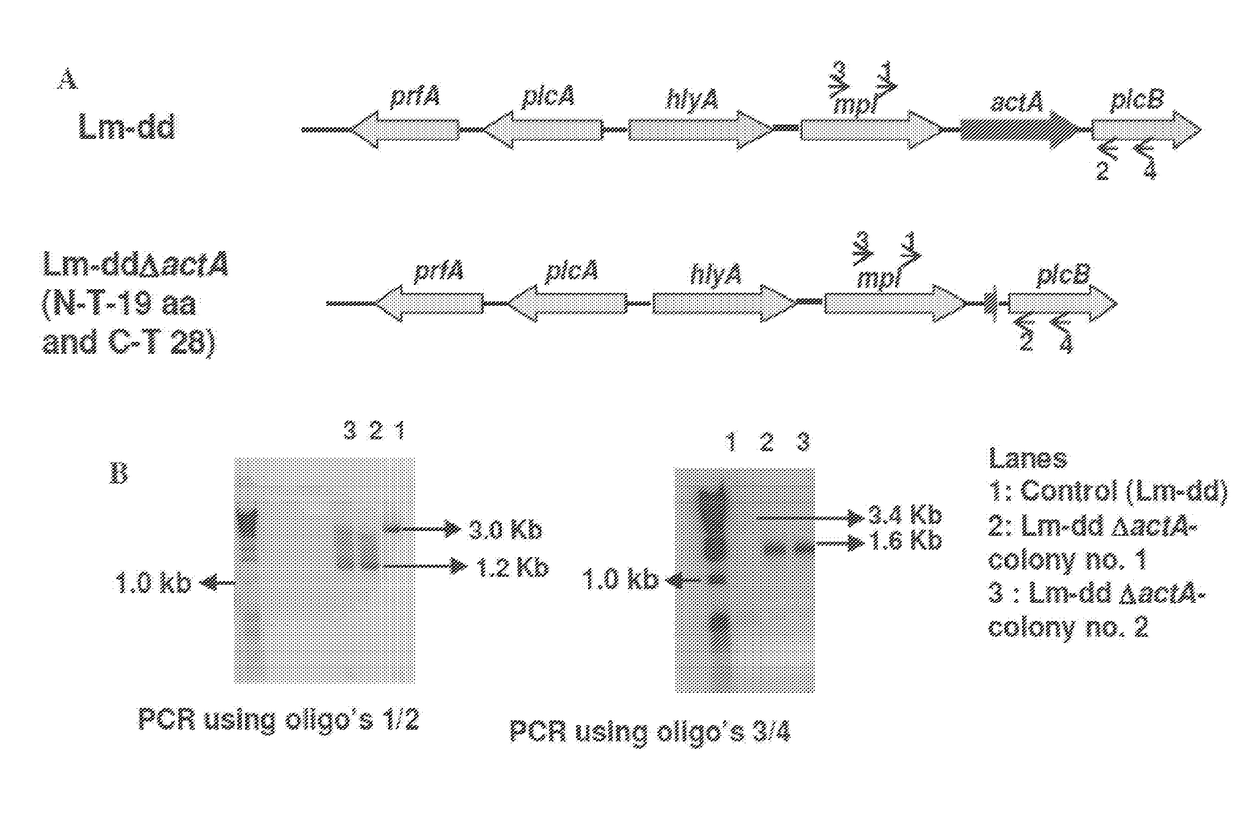
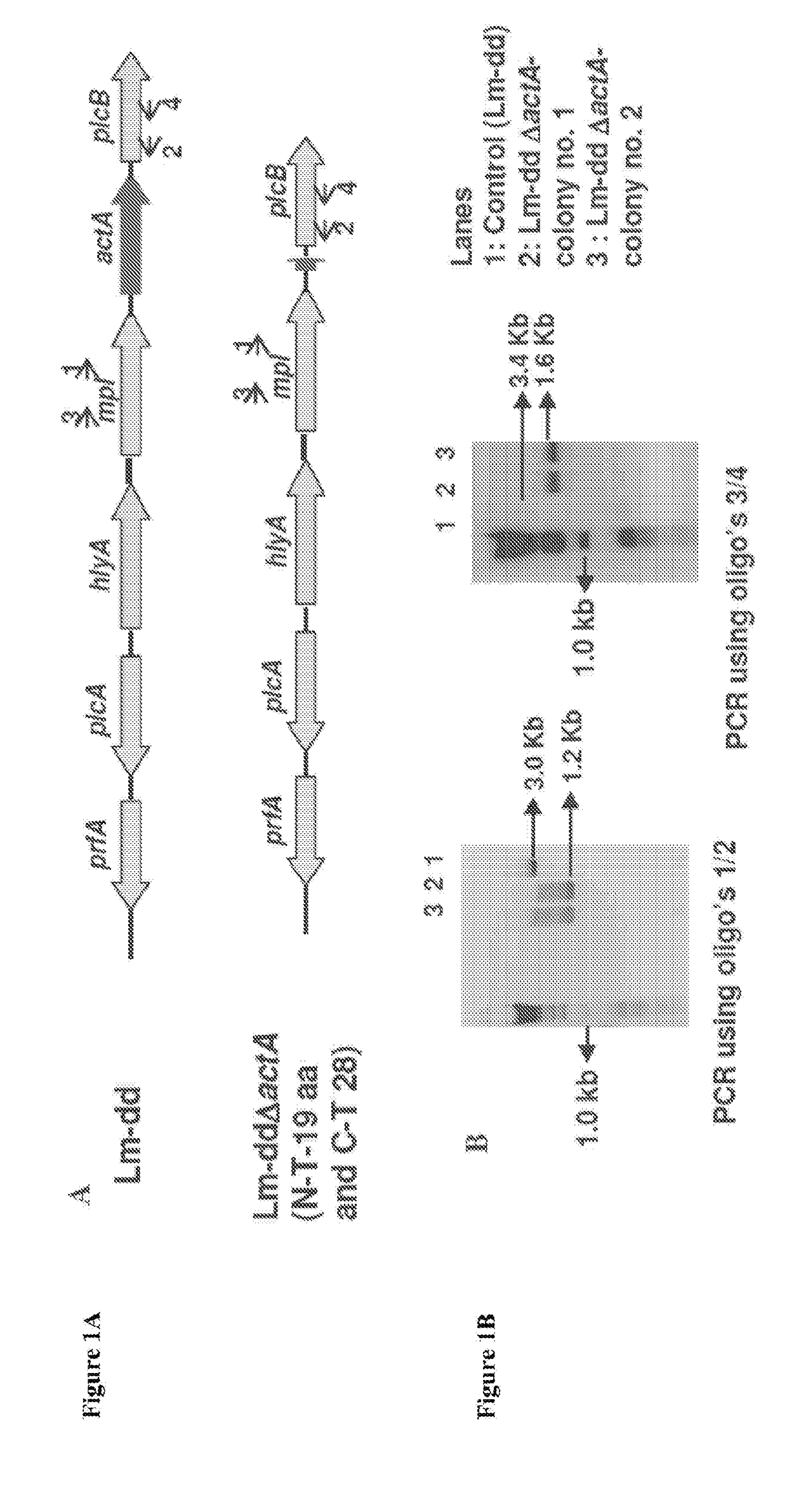
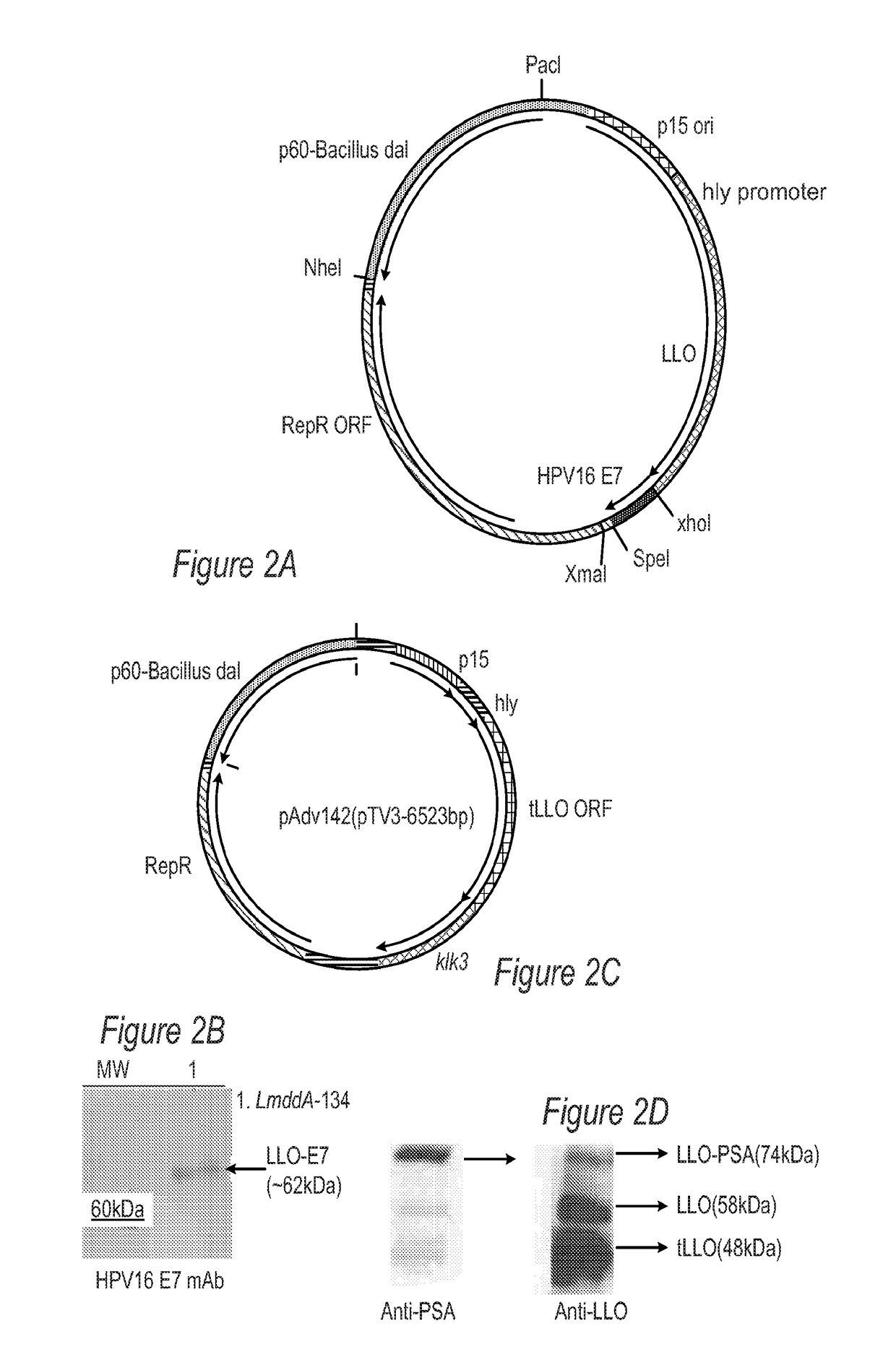
The disclosure is directed to compositions comprising an oncolytic virus, chimeric antigen receptor T cells (CAR T cells), a therapeutic or immunomodulating monoclonal antibody, a targeting thymidine kinase inhibitor (TKI), or an adoptively transferred cells incorporating engineered T cell receptors, and a live attenuated recombinant Listeria strain comprising a fusion protein of a Truncated LLO, a truncated ActA or a PEST-sequence peptide fused to a tumor-associated antigen. The disclosure is further directed to methods of treating, protecting against, and inducing an immune response against a tumor, comprising the step of administering the same, with or without an additional radiation therapy treatment.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
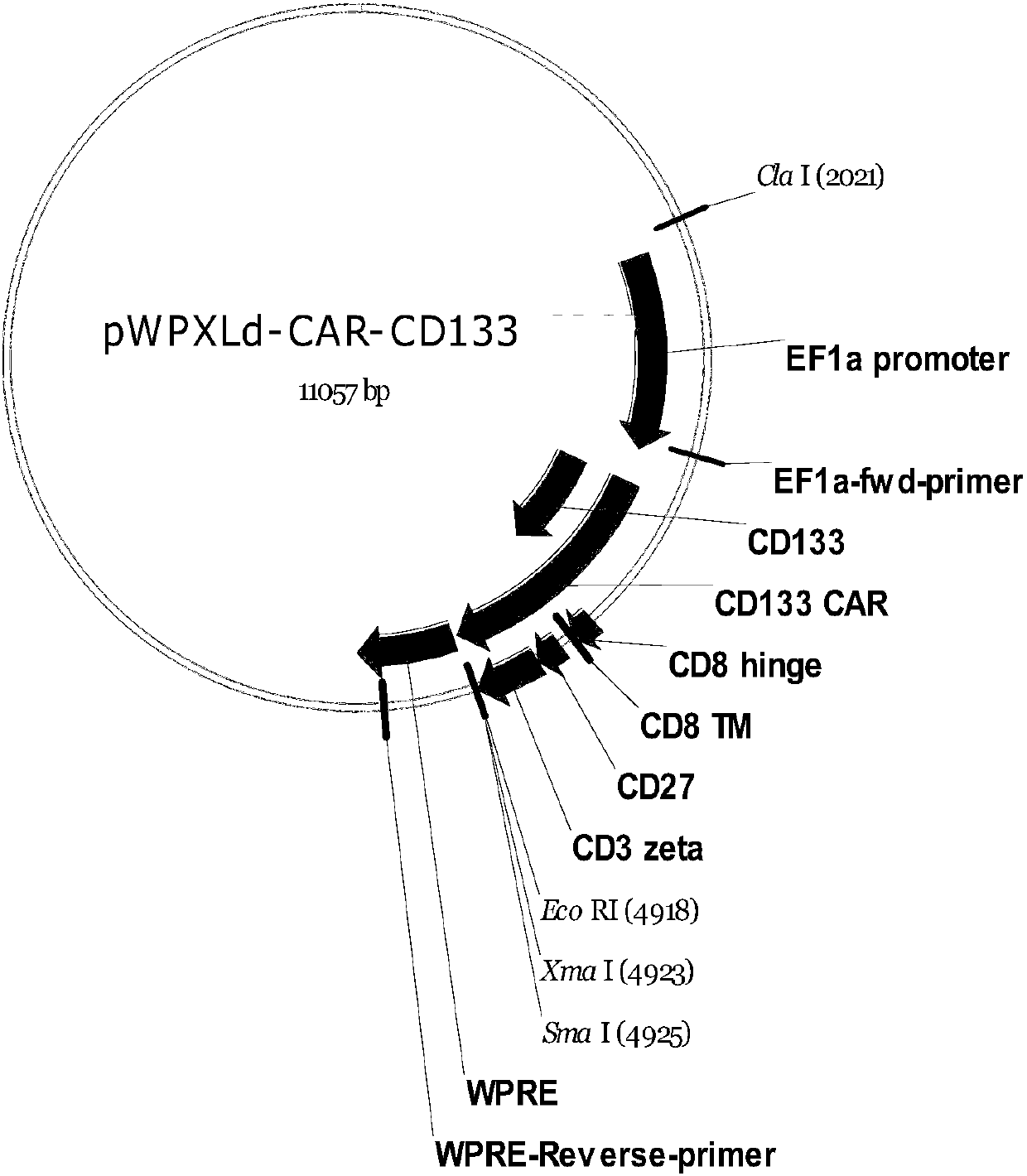
Chimeric antigen receptor and chimeric antigen receptor T cells targeting CD133, preparation method of chimeric antigen receptor T cells and application of chimeric antigen receptor and chimeric antigen receptor T cells
InactiveCN110526987AImprove efficiencyEfficient transferMammal material medical ingredientsImmunoglobulinsSingle-Chain AntibodiesHinge region
The invention provides a chimeric antigen receptor CAR-CD133 targeting CD133 and chimeric antigen receptor T cells targeting the CD133. The CAR-CD133 comprises amino acid sequences of a single-chain antibody targeting CD133, an extracellular hinge region, a transmembrane region and an intracellular signal region which are sequentially connected from an amino terminal to a carboxyl terminal. The CAR-CD133 can specifically target CD133, takes a CD27 signal region as a co-stimulation signal region, activates T cells to play a role in cellular immunity, promotes amplification of the T cells in a patient body, efficiently and specifically kills CD133 positive tumor cells, and has lasting cell viability and lethality. The invention also provides a preparation method of the chimeric antigen receptor T cells targeting the CD133, a recombinant virus vector with the CAR-CD133 coding gene, and the like.
Owner:SHENZHEN BINDEBIOTECH CO LTD
Chimeric antigen receptor T cell targeting CD317, and preparation method and application thereof
InactiveCN109957546APromote amplificationImprove acceleration performanceAntibody mimetics/scaffoldsMammal material medical ingredientsSingle-Chain AntibodiesHinge region
The present invention provides a chimeric antigen receptor T cell targeting CD317. The chimeric antigen receptor T cell targeting the CD317 comprises a chimeric antigen receptor CAR-CD317 targeting the CD317, the CAR-CD317 comprises amino acid sequences of a single-chain antibody targeting the CD317, an extracellular hinge region, a transmembrane region, and an intracellular signal region with anamino terminus successively connected to a carboxy terminus, the single-chain antibody targeting the CD317 has the amino acid sequence shown in SEQ ID NO:1, the intracellular signal region comprises aCD28 signal region and a CD3 zeta signal region in a successive connection, and the CD28 signal region comprises the amino acid sequence shown in SEQ ID NO:2. The CAR-CD317 can specifically target the CD317, promote proliferation of T cells in patient body, and efficiently and specifically kill tumor cells. The present invention also provides a preparation method and an application of the T cell.
Owner:SHENZHEN BINDEBIOTECH CO LTD
Chimeric antigen receptor therapy with reduced cytotoxicity for viral disease
ActiveUS20190307798A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenFc(alpha) receptor
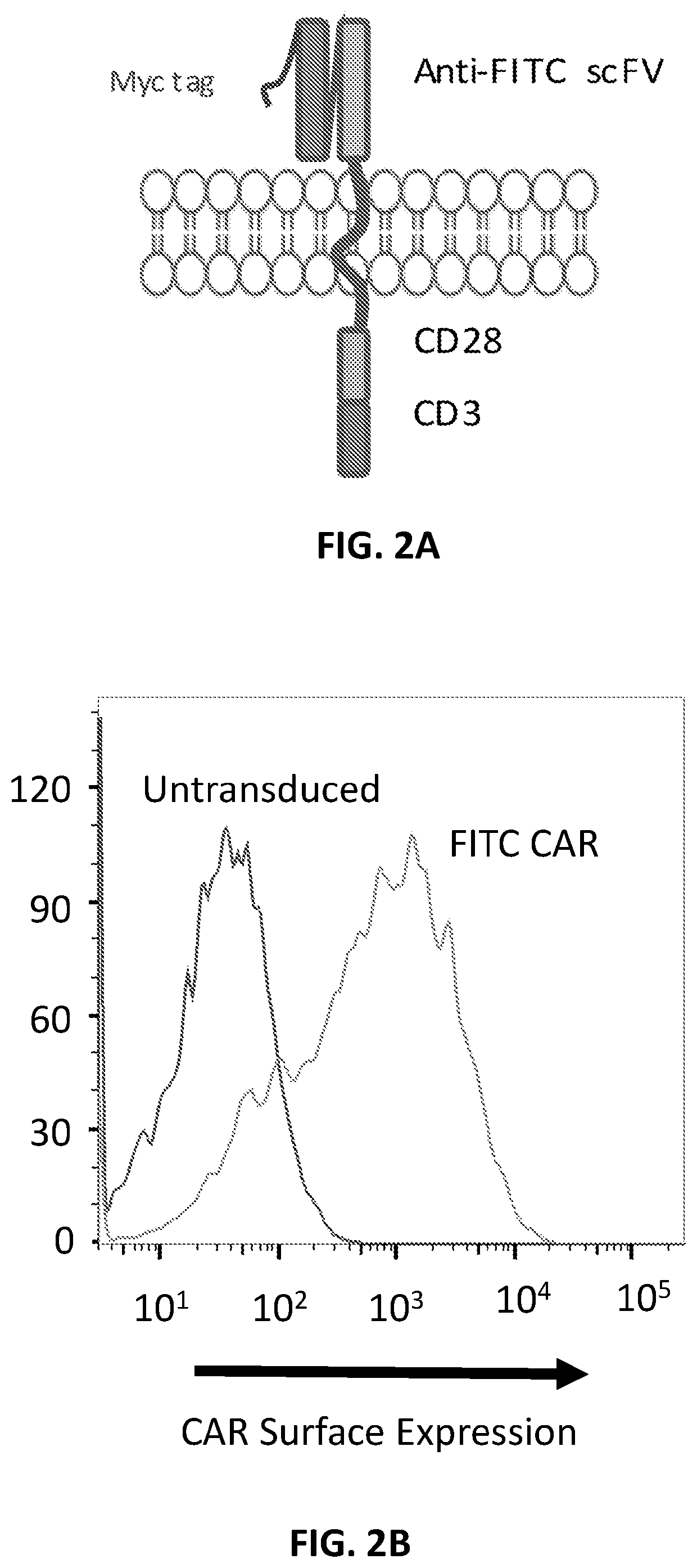
Embodiments of the disclosure encompass immunotherapy for Hepatitis B viral (HBV) infection in an individual in need thereof. The immunotherapy comprises one or more chimeric antigen receptors (CAR) that target a HBV antigen, including CAR molecules that utilize specific scFv antibodies. In certain cases, the CAR comprises one or more mutations to reduce binding to Fc receptors. In specific aspects, cells that express the CAR(s) have reduced cytotoxicity that is safer and / or beneficial to individuals that are immunocompromised.
Owner:BAYLOR COLLEGE OF MEDICINE
Compositions for chimeric antigen receptor t cell therapy and uses thereof
InactiveUS20200230221A1Expands CAR-TFunction increaseTumor rejection antigen precursorsAntibody mimetics/scaffoldsHydrophilic polymersAntigen receptors
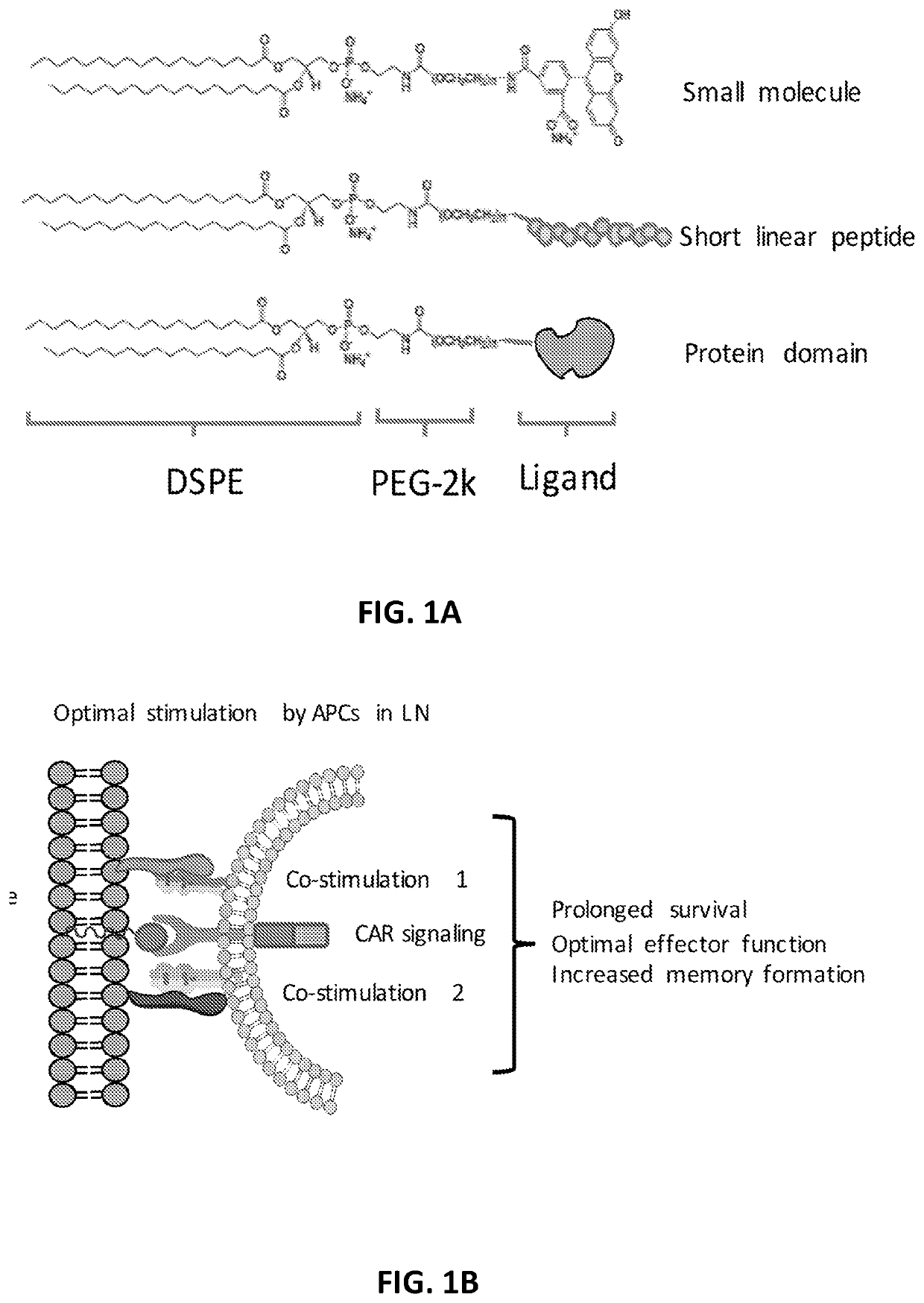
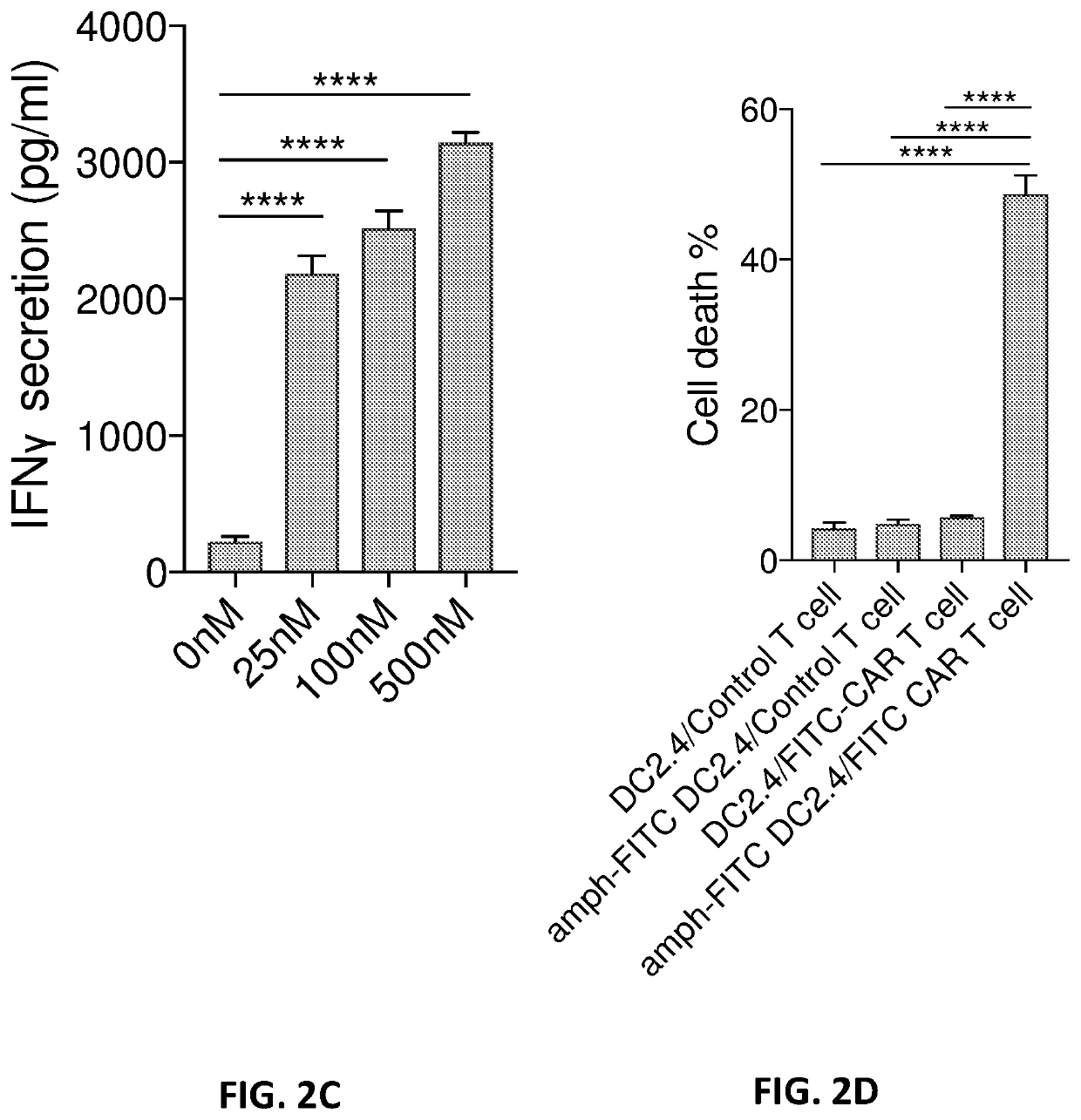
The disclosure describes amphiphilic ligand conjugates comprising a chimeric antigen receptor (CAR) ligand, a lipid (diacyl lipid), a linker (hydrophilic polymers, hydrophilic amino acids, polysaccharides), compositions and methods of using the constructs are claimed, for example, to stimulate proliferation of CAR expressing cells.
Owner:MASSACHUSETTS INST OF TECH
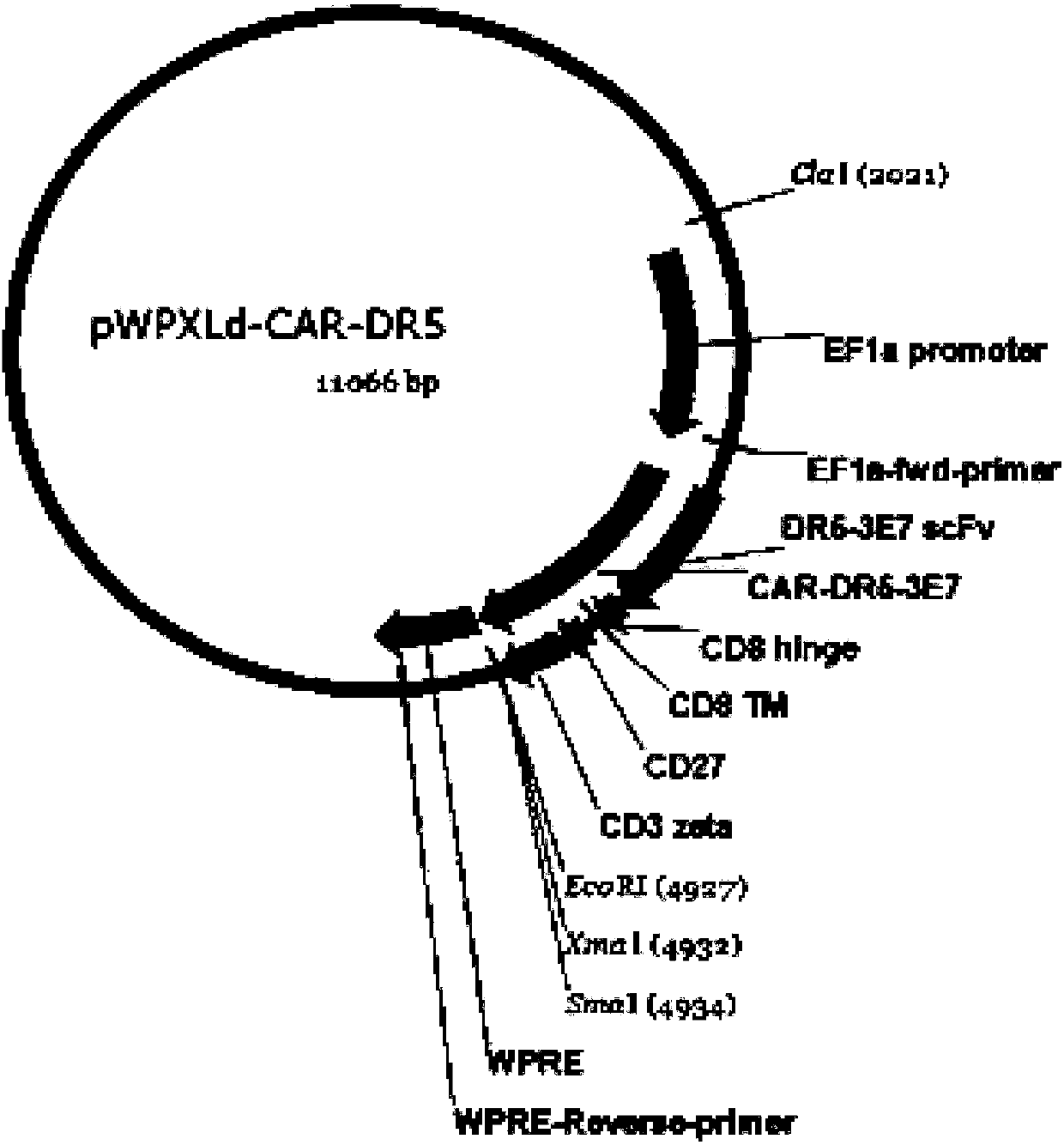
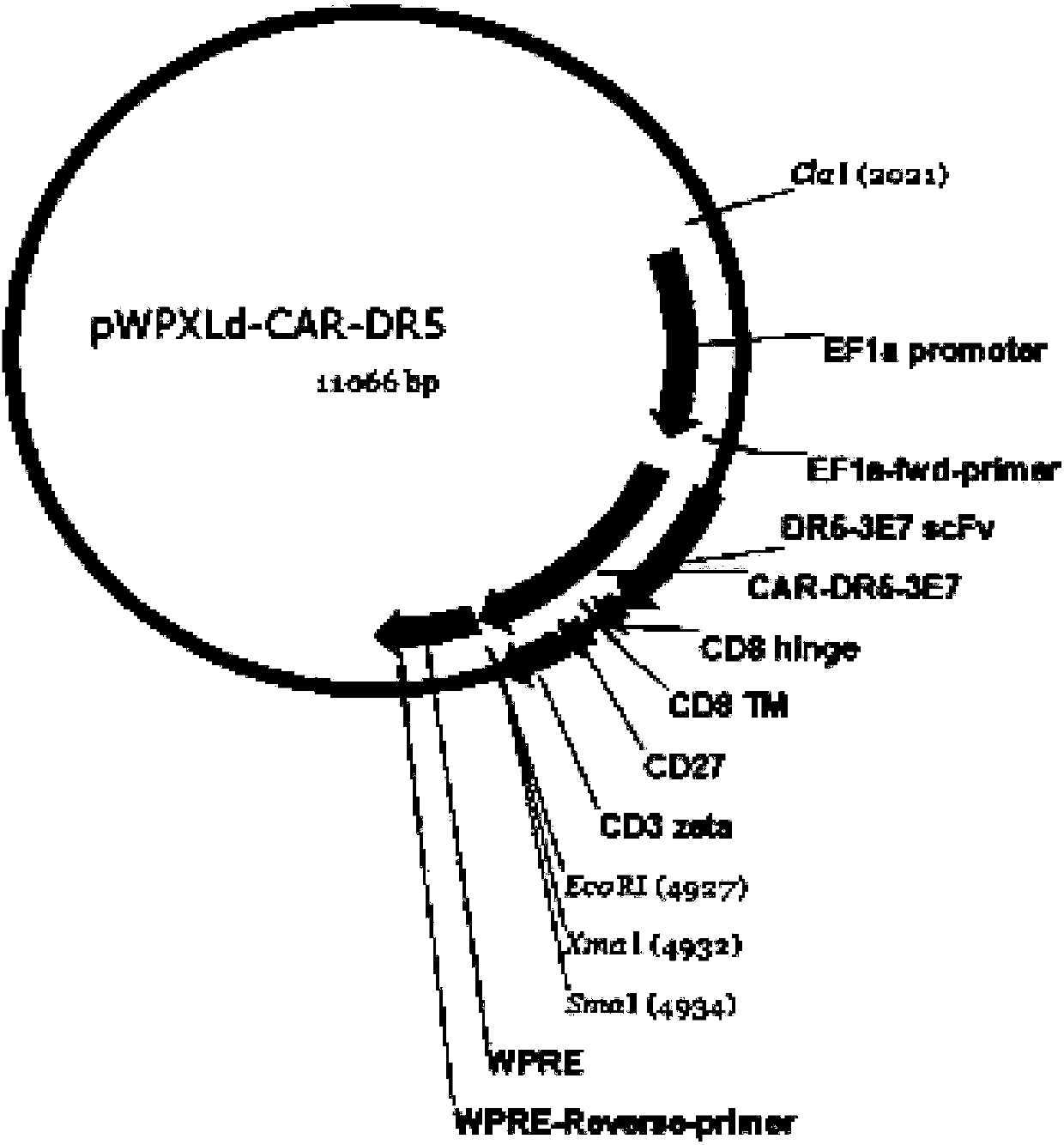
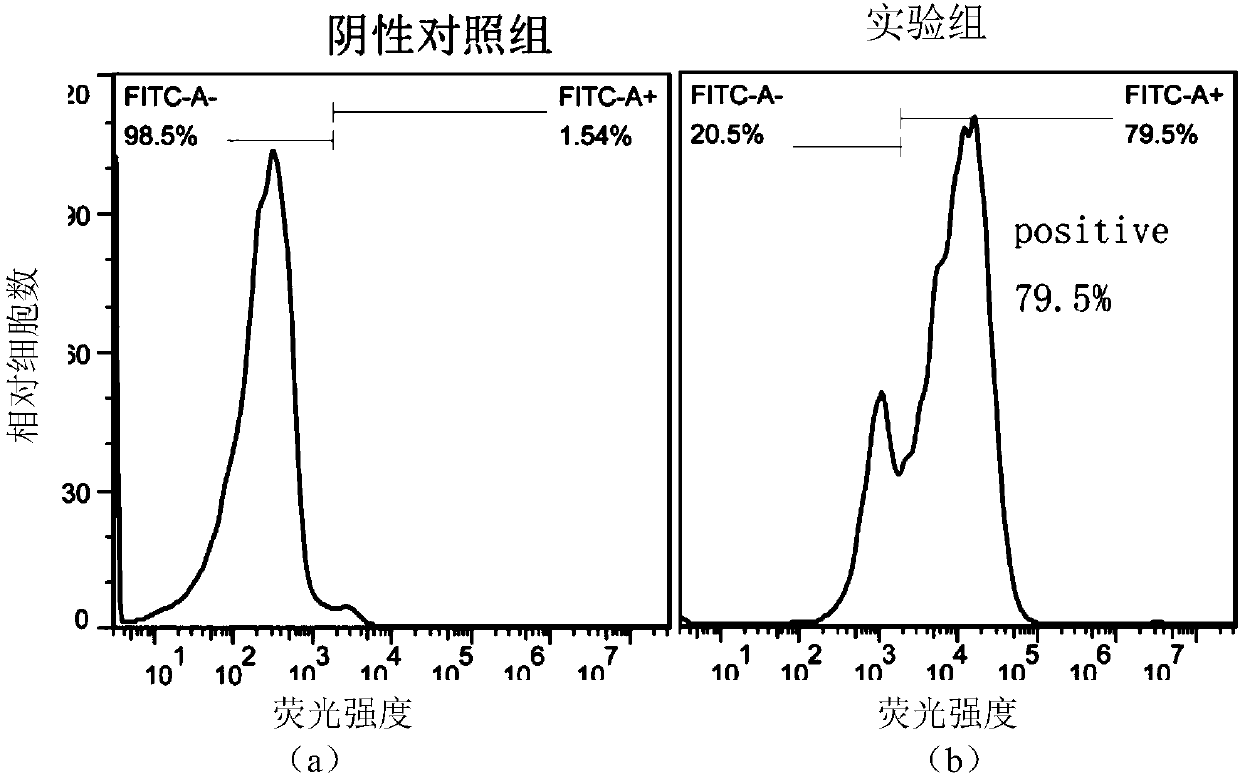
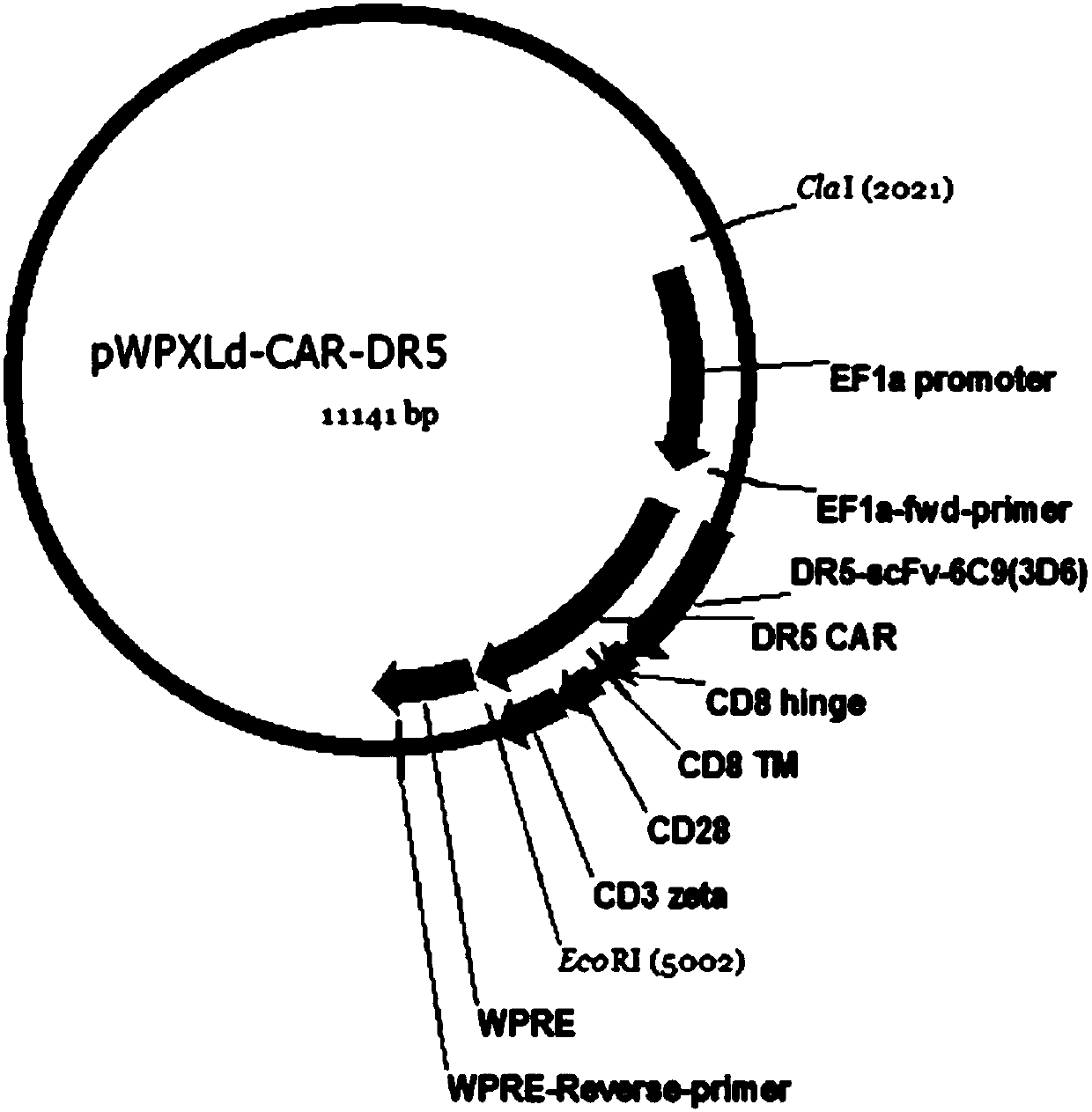
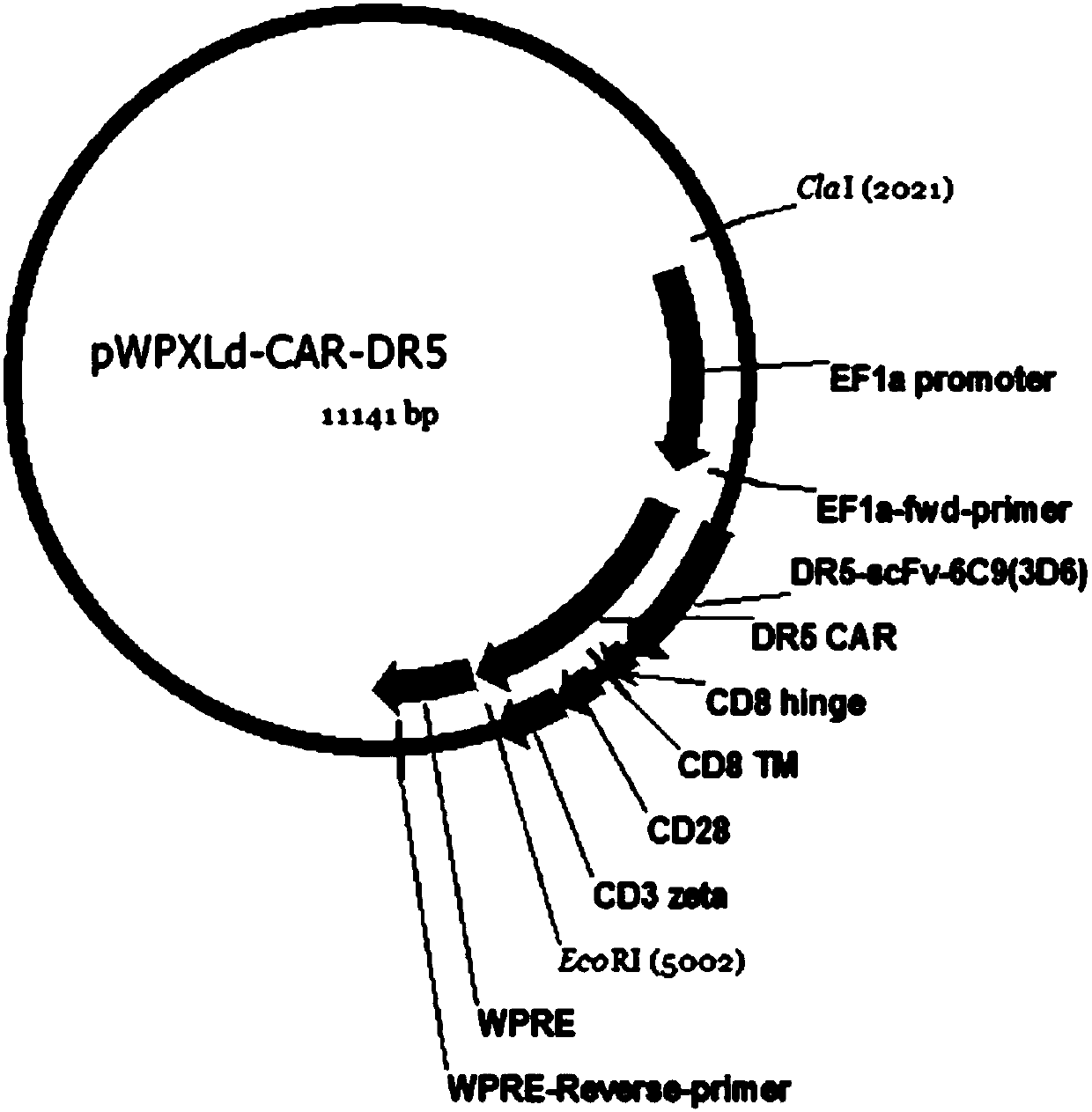
DR5-targeting single-chain antibody, chimeric antigen receptor T cell, preparation method and application of of chimeric antigen receptor T cell
InactiveCN109957025AHigh affinityPromote amplificationAntibody mimetics/scaffoldsMammal material medical ingredientsAntigenSingle-Chain Antibodies
The present invention provides a DR5-targeting single-chain antibody, comprising an amino acid sequence shown in SEQ ID NO: 1. The invention also provides a chimeric antigen receptor T cell comprisingthe DR5-targeting single-chain antibody, and the DR5-targeting chimeric antigen receptor can specifically target DR5 to promote amplification of T cell in the body of a patient, thus efficiently andspecifically kill tumor cells and preferably maintain viability and killing effect of cells without damaging normal cells. The invention further provides a preparation method and application of the DR5-targeting chimeric antigen receptor T cell.
Owner:SHENZHEN BINDEBIOTECH CO LTD
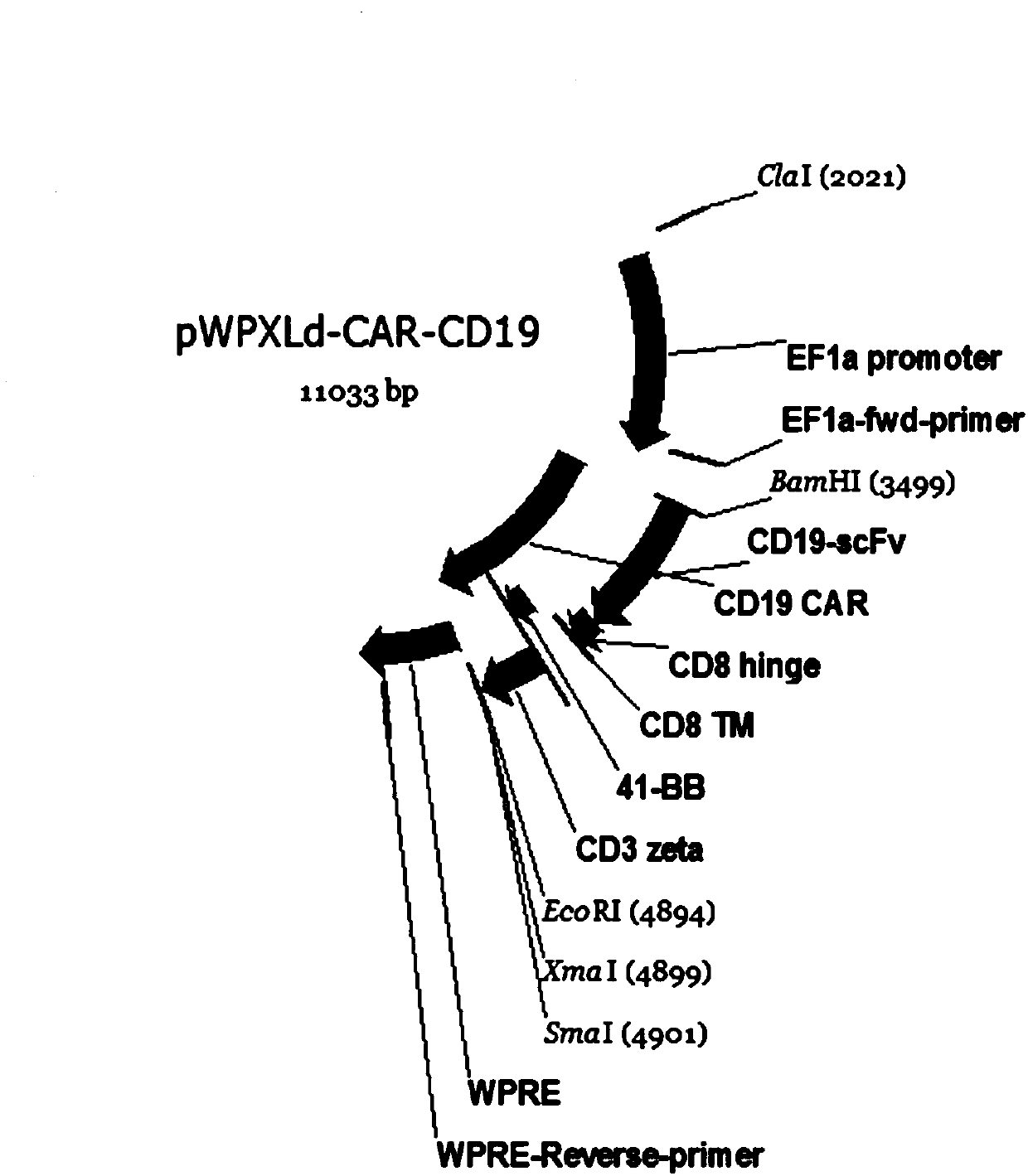
Single-chain antibody targeting CD19, chimeric antigen receptor T cell, preparation method and application thereof
InactiveCN109836493APromote amplificationEfficient and specific killingImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationSingle-Chain AntibodiesT cell
The present invention provides a single-chain antibody targeting CD19, the single-chain antibody targeting CD19 comprises an amino acid sequence shown in SEQ ID NO: 1, and the single-chain antibody targeting CD19 is a humanized single-chain antibody. The present invention also provides a chimeric antigen receptor T cell comprising the single-chain antibody targeting CD19, wherein the chimeric antigen receptor targeting CD19 can specifically target CD19 on tumor cells, the expansion of T cells in patients is promoted, and the tumor cells can be efficiently and specifically killed. In addition,the chimeric antigen receptor T cell targeting CD19 produced by humanized single-chain antibody has better cell viability and lethality. The invention also provides a preparation method and an application of the chimeric antigen receptor T cell targeting CD19.
Owner:SHENZHEN BINDEBIOTECH CO LTD
Application of Lactobacillus paracasei to CAR (chimeric antigen receptor)-T cell therapy
ActiveCN110305821AStrong immunomodulatory activityEnhance immune functionAntibacterial agentsBacteriaMicroorganismTumor therapy
The invention relates to an application of Lactobacillus paracasei to CAR (chimeric antigen receptor)-T cell therapy, belongs to the technical field of microorganisms and particularly discloses Lactobacillus paracasei MIT-16 and an application of the Lactobacillus paracasei to the immunological therapy process of canine tumors. The Lactobacillus paracasei MIT-16 with higher acid-producing abilityis separated and screened from an intestinal tract of a mouse, has good acid resistance, stronger bactericidal capacity and remarkable immune regulation functions, particularly plays an important rolein immune response regulation in tumor therapy and can be used as an immune regulation biotherapy agent.
Owner:LUDONG UNIVERSITY
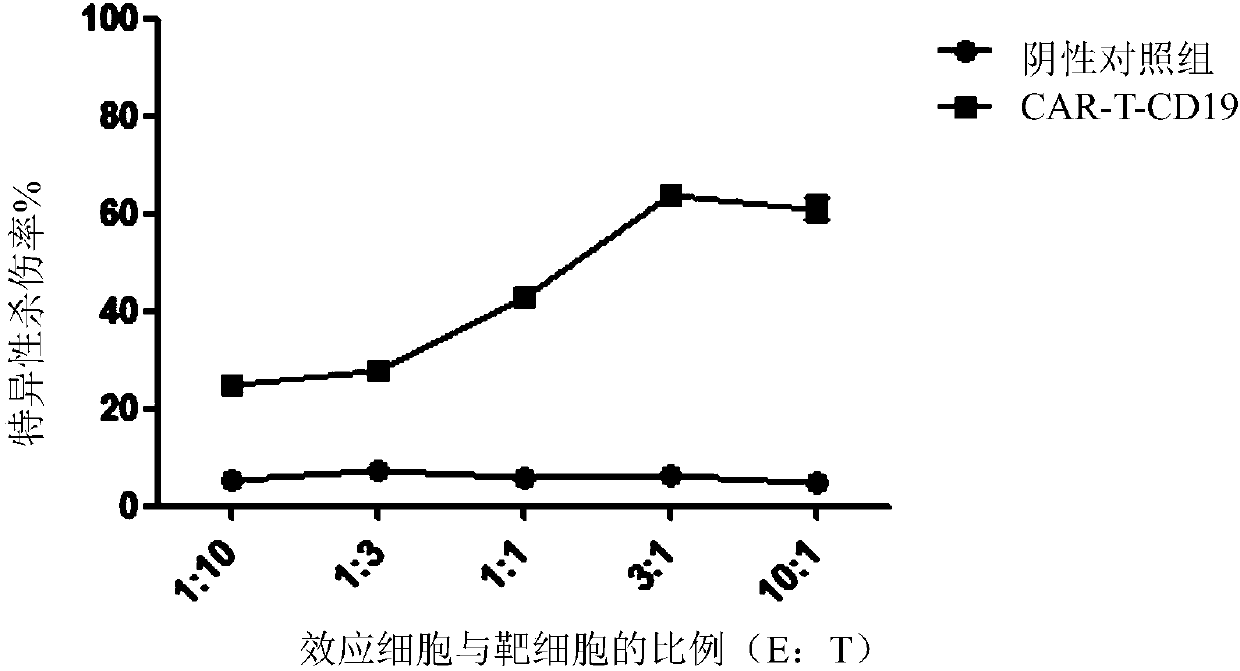
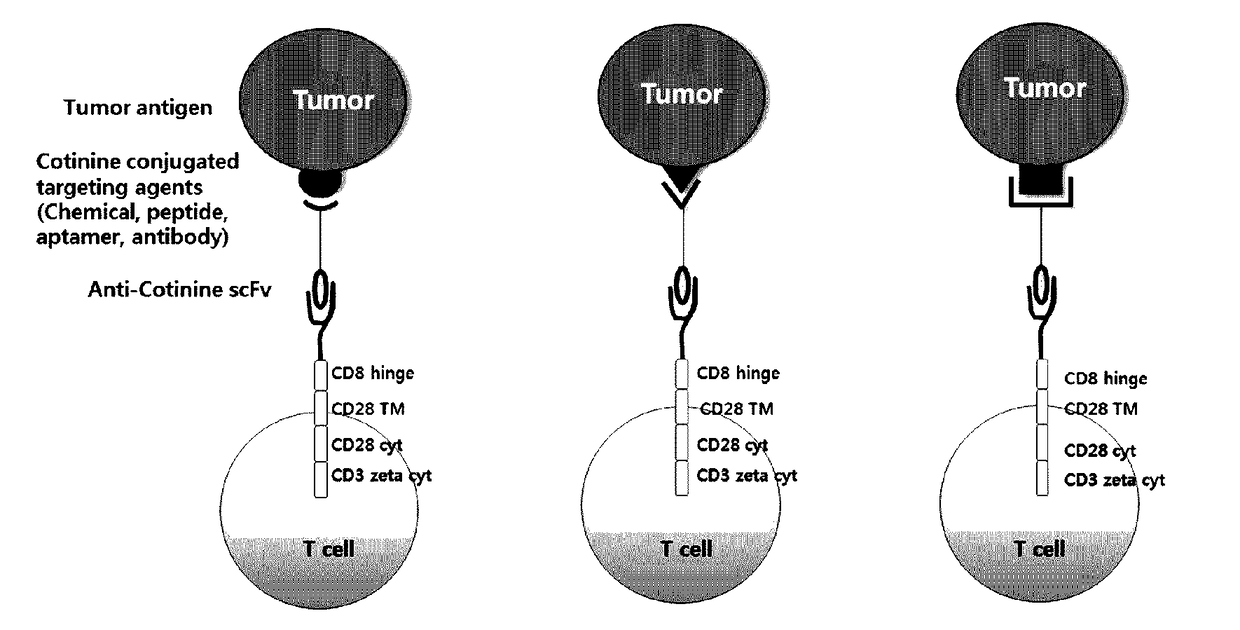
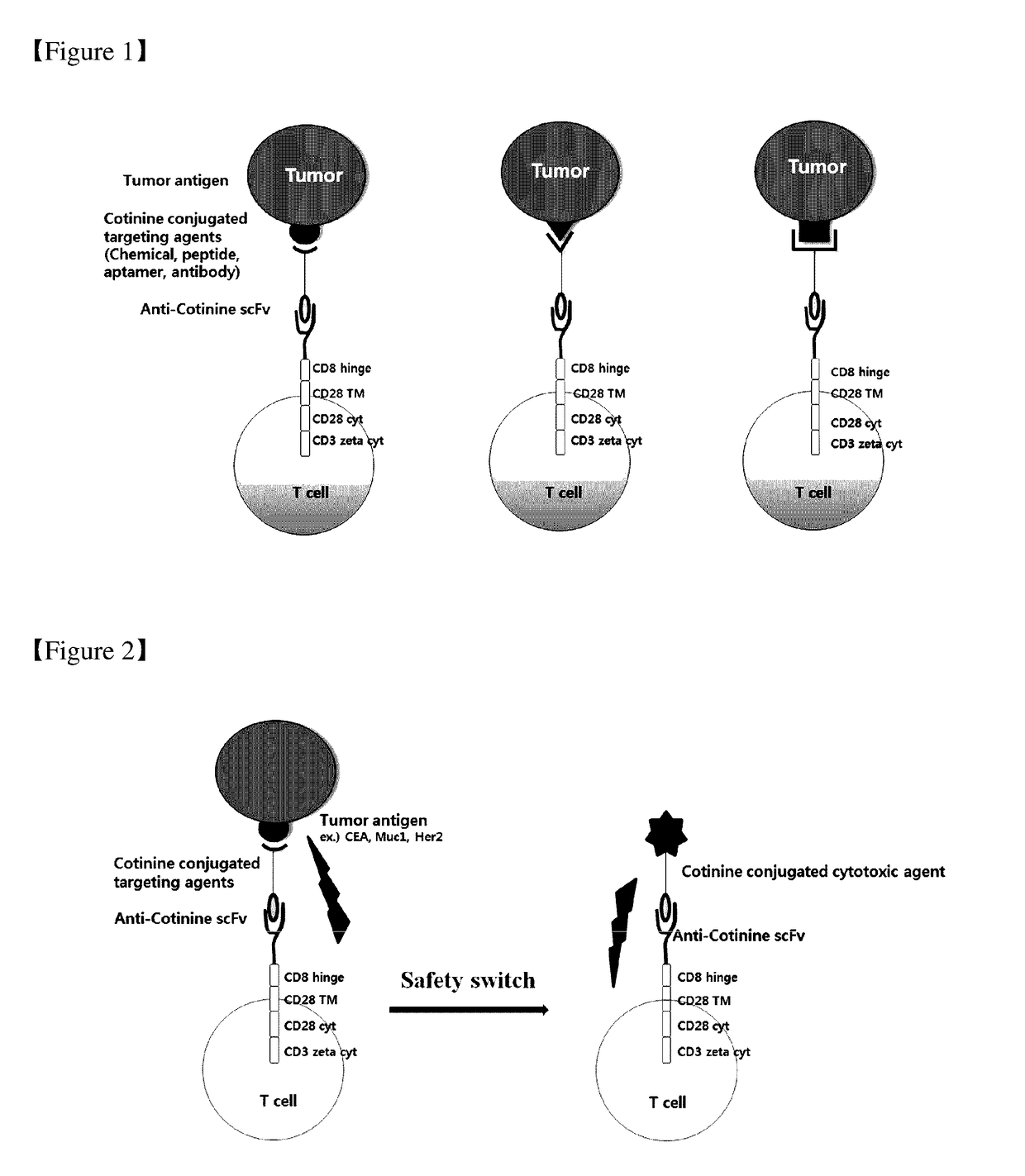
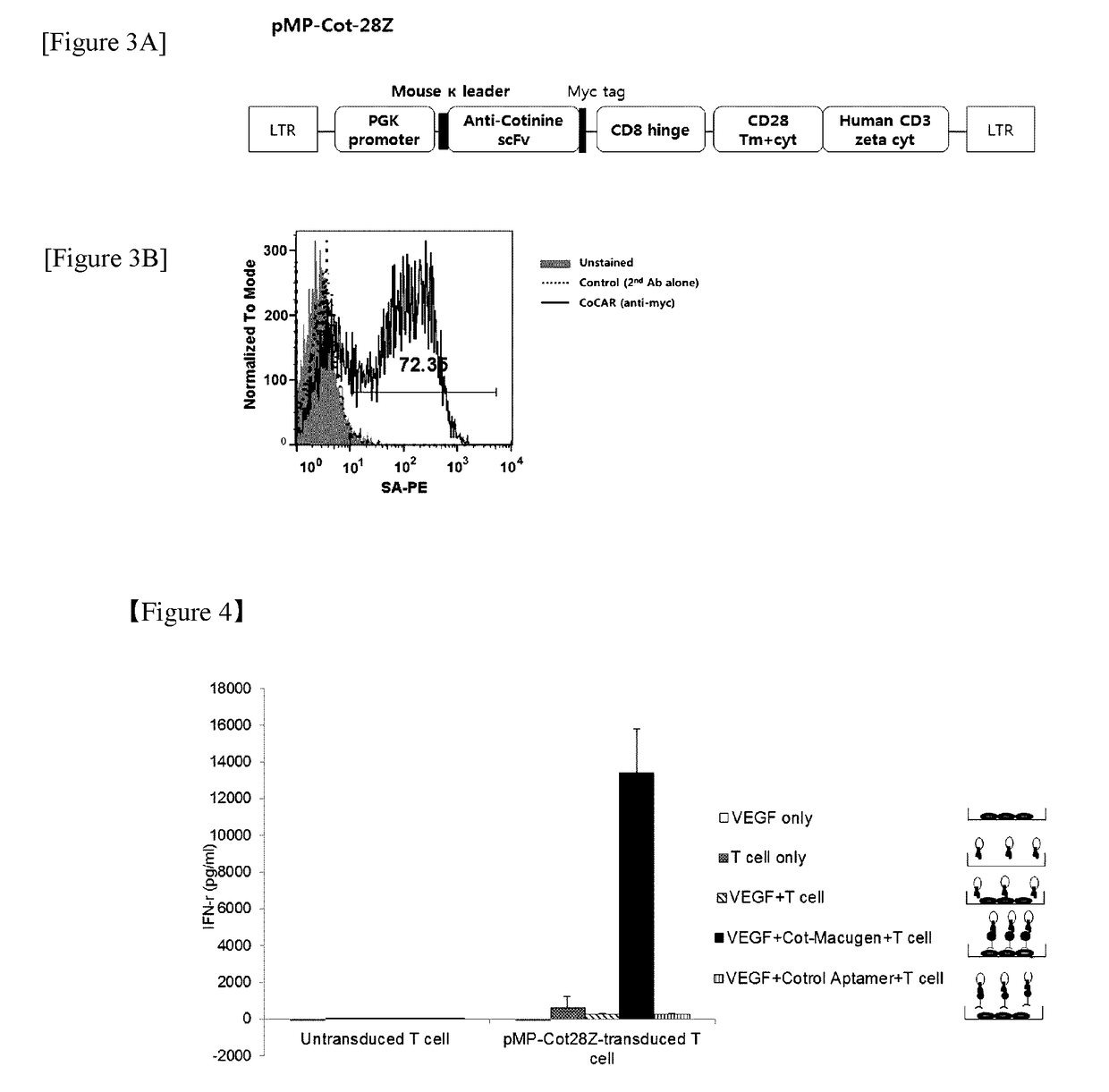
Chimeric antigen receptor to which Anti-cotinine antibody is linked, and use thereof
ActiveUS20180256744A1Induce cell deathSuppress adverse immune side effectOrganic active ingredientsPeptide/protein ingredientsAntigenCotinine
The present invention relates to chimeric antibody receptors with anti-cotinine antibodies linked, and use thereof. A T cell presenting the chimeric antibody receptor on the surface secretes interferon gamma specifically for a target molecule of a cotinine-conjugated binding molecule that is added together therewith and induces cell death of the cell expressing the target molecule by the T cell. On the contrary, by administering a cytotoxic agent conjugated with cotinine, cell death of the chimeric antigen receptor T cell is induced. Therefore, if necessary, a cytotoxic agent conjugated with cotinine can be administered to remove the chimeric antigen receptor T cells that have been already administered, thereby suppressing immune side effects due to hyperactivity of T cells. Thus, the chimeric antigen receptor to which the anti-cotinine antibody is linked can be effectively and safely used for the treatment of cancer.
Owner:SEOUL NAT UNIV R&DB FOUND
Single-chain antibody and chimeric antigen receptor T cells targeting DR5, and preparation method and application thereof
InactiveCN109957020AHigh affinityPromote amplificationPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenSingle-Chain Antibodies
The present invention provides a single-chain antibody targeting DR5. The single-chain antibody targeting the DR5 comprises an amino acid sequence shown as SEQ ID NO:1. The present invention also provides chimeric antigen receptor T cells comprising the single-chain antibody targeting the DR5. Chimeric antigen receptors targeting the DR5 can specifically target the DR5, promote T cell proliferation in patient body, efficiently and specifically kill tumor cells, can better maintain cell viability and lethality, and also do not cause damages on normal cells. The present invention also provides apreparation method and an application of the chimeric antigen receptor T cells targeting the DR5.
Owner:SHENZHEN BINDEBIOTECH CO LTD
Use of endogenous viral vaccine in chimeric antigen receptor T cell therapy
ActiveUS11116834B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyViral VaccineTGE VACCINE
Provided herein are, inter alia, methods and compositions including T cells expressing (i) a recombinant CAR protein which includes a peptide binding site and is capable of specifically binding cancer-specific antigens and (ii) a T cell receptor specific for a viral antigen (e.g., a CMV pp65 protein). The engineered T cells provided herein may be used in combination with a viral vaccine (e.g. cytomegalovirus (CMV) Triplex Vaccine) to treat a variety of cancers. The methods described herein also permit in viva expansion of CMV-specific CAR T cells, instead of or in addition to ex vivo expansion, avoiding excessive T cell exhaustion that results in some cases from ex vivo manufacturing.
Owner:CITY OF HOPE
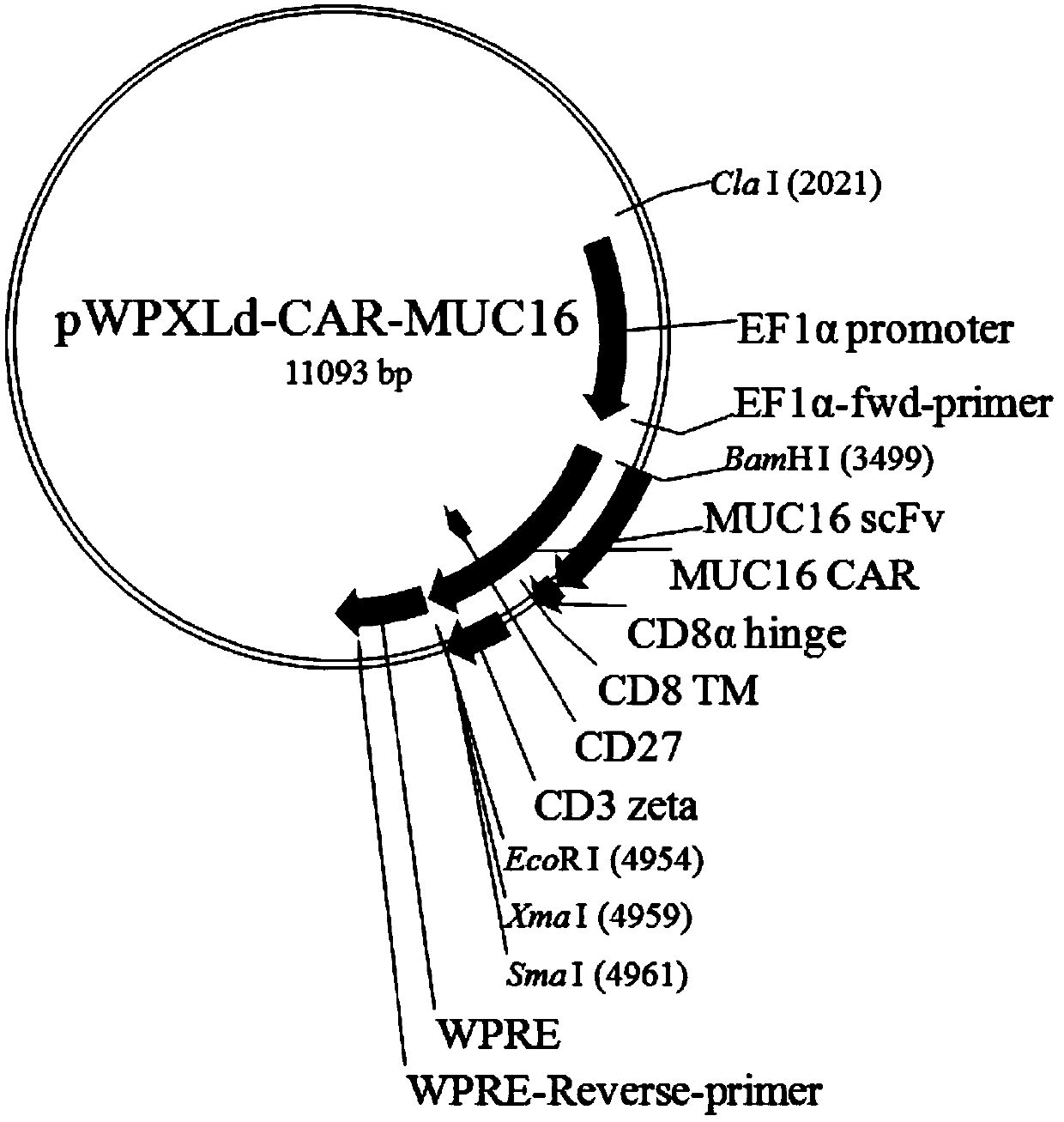
MUC16-targeting single-strand antibody, and MUC16-targeting chimeric antigen receptor T cell and preparation method and application thereof
InactiveCN110526974AHigh affinityPromote amplificationPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenSingle-Chain Antibodies
The invention provides an MUC16-targeting single-strand antibody. The amino acid sequence of the MUC16-targeting single-strand antibody comprises an amino acid sequence as shown in SEQ ID NO:1. The invention further provides a chimeric antigen receptor T cell including the MUC16-targeting single-strand antibody. The MUC16-targeting chimeric antigen receptor can specifically target MUC16, can promote amplification of T cells in patient bodies, can efficiently and specifically kill tumor cells and can better maintain vitality and lethality of the cells. The invention further provides a preparation method and application of the MUC16-targeting chimeric antigen receptor T cell.
Owner:SHENZHEN BINDEBIOTECH CO LTD
A chimeric antigen receptor NK cell and a preparation method and an application thereof
InactiveCN109207430AIncrease lethalityImprove anti-tumor activityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyNatural Killer Cell Inhibitory ReceptorsFungating tumour
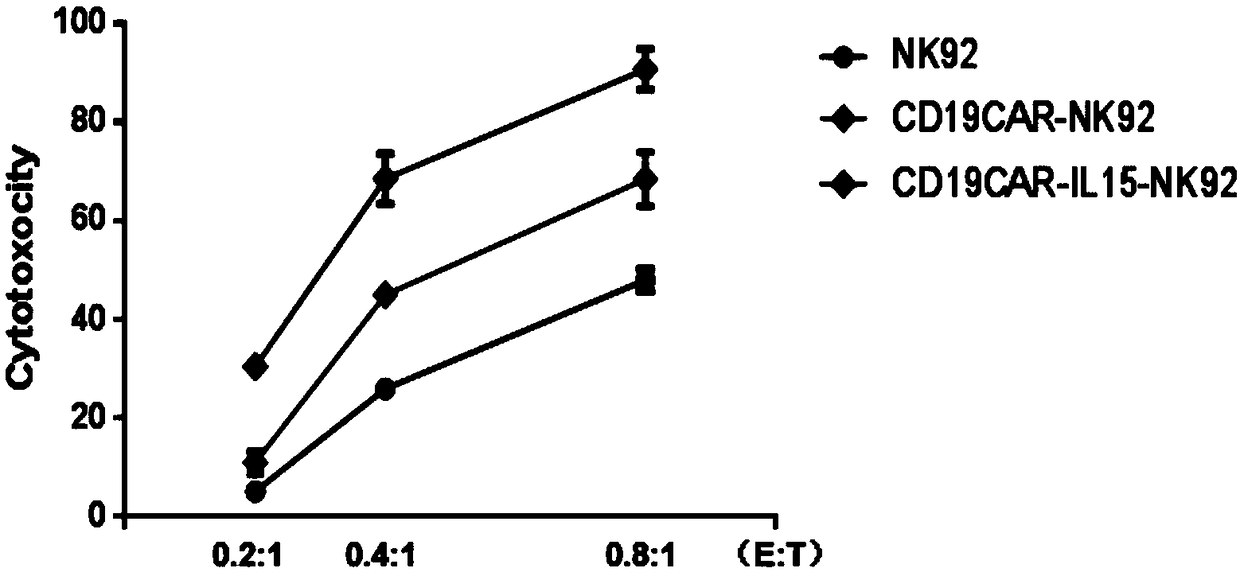
The invention discloses a chimeric antigen receptor NK cell, a preparation method and an application thereof, which relate to the technical field of cellular immunotherapy. The chimeric antigen receptor NK cell disclosed by the invention can simultaneously express IL-15 and a chimeric antigen receptor targeting CD19. The chimeric antigen receptor NK cells have good killing effect on tumor cells, and can be used for preparing anti-tumor drugs, thus providing a new idea and method for treating tumor.
Owner:EAST CHINA NORMAL UNIVERSITY +1
CD30 targeted chimeric antigen receptor, and CD30 targeted chimeric antigen receptor T cell and preparation method and application thereof
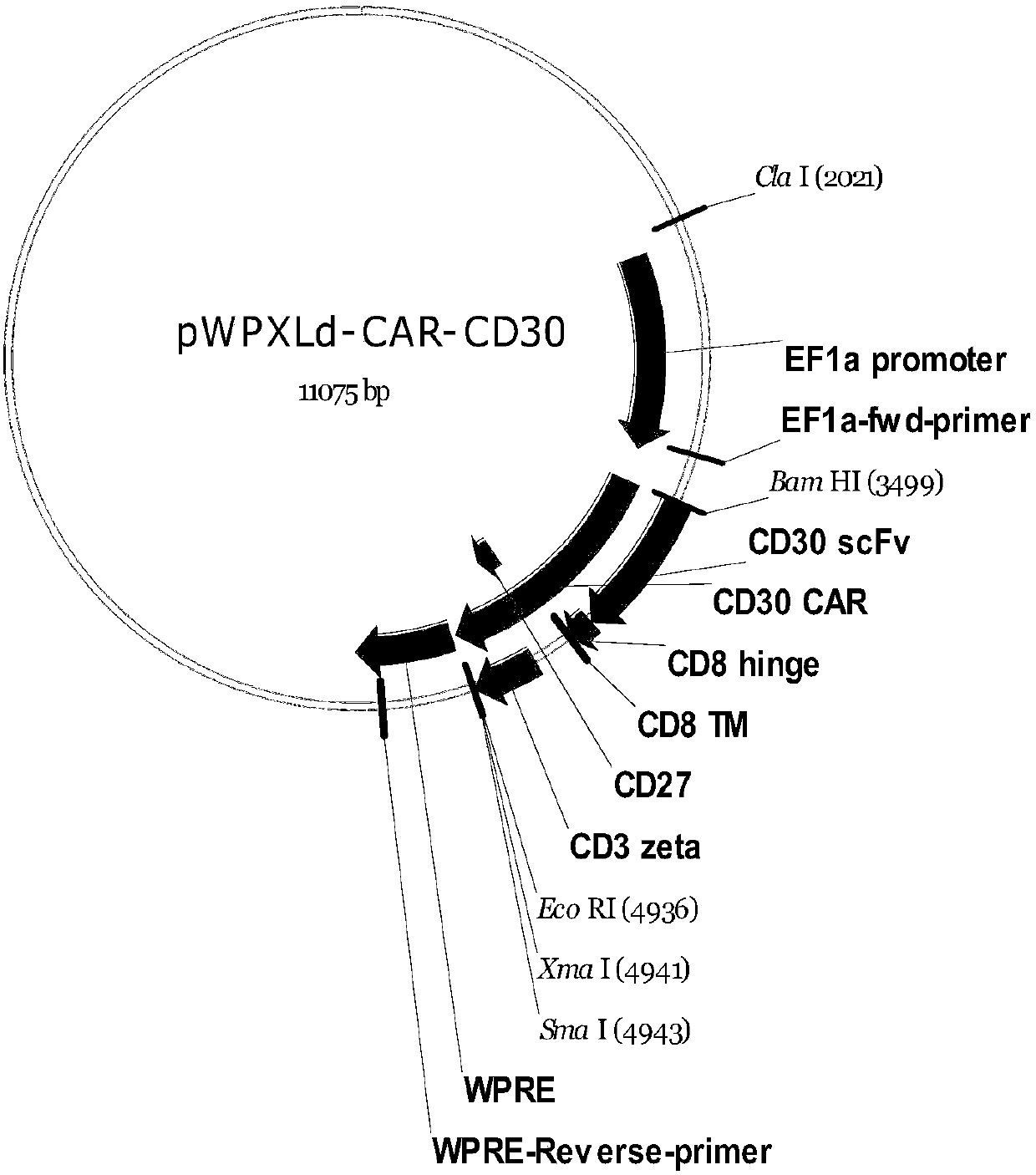
The invention provides a CD30 targeted chimeric antigen receptor CAR-CD30. The CAR-CD30 comprises an amino acid sequence as shown in SEQID NO:1. The invention further provides a chimeric antigen receptor T cell including the CAR-CD30. The CD30 targeted chimeric antigen receptor CAR-CD30 can specifically target CD30 positive tumor cells and activate T cells to exert cellular immunity effects to realize efficient and specific killing on the CD30 positive tumor cells, has persistent cell viability and lethality, and cannot damage normal cells. The invention further provides a preparation method of the CD30 targeted chimeric antigen receptor T cell, a recombinant virus vector having CAR-CD30 coding genes, and the like.
Owner:SHENZHEN BINDEBIOTECH CO LTD
HPK1-targeted gRNA and editing method of HPK1 gene
ActiveCN109517820AHigh knockout efficiencyEasy to operateHydrolasesAntibody mimetics/scaffoldsPeripheral blood mononuclear cellCell tumor
The invention relates to HPK1-targeted gRNA and an editing method aiming at an HPK1 gene. According to HPK1gRNA and the editing method of the HPK1 gene, the HPK1 gene of the T cell can be knocked out,the killing activity of the T cell can be improved, the Th1 cell factor level of a peripheral blood monouclear cell can be increased; and by knocking out the HPK1 gene of the T cell, the expression of PD-1 and TIM3 on the surface of the T cell can be decreased, and the failure of the T cell can be inhibited. Therefore, HPK1-targeted gRNA can be used for preparing tumor treatment drug and can be particularly applied to targeting therapy methods (CRA-T, CAR-NK, CAR-NKT and CAR-gammadelta) for chimeric antigen receptor cell tumors.
Owner:BEIJING YUFAN BIOTECH CO LTD
CD317-targeting chimeric antigen receptor T cell as well as preparation method and application thereof
InactiveCN109957547APromote amplificationImprove acceleration performancePolypeptide with localisation/targeting motifImmunoglobulin superfamilySingle-Chain AntibodiesHinge region
The invention provides a CD317-targeting chimeric antigen receptor T cell. The CD317-targeting chimeric antigen receptor T cell comprises CD317-targeting chimeric antigen receptor CAR-CD317, the CAR-CD317 comprises amino acid sequences of a CD317-targeting single-chain antibody, an extracellular hinge region, a transmembrane region and an intracellular signal region which are sequentially connected from an amino terminal to a carboxyl terminal, the CD317-targeting single-chain antibody comprises an amino acid sequence shown in SEQ ID NO:1, the intracellular signal region comprises a CD27 signal region and a CD3zeta signal region, and the CD27 signal region comprises an amino acid sequence shown in SEQ ID NO:2. The CAR-CD317 can specifically target CD317 promote amplification of the T cellin a patient and efficiently and specifically kill tumor cells. The invention also provides a preparation method and an application of the T cell.
Owner:SHENZHEN BINDEBIOTECH CO LTD
MUC16 targeted chimeric antigen receptor and MUC16 targeted chimeric antigen receptor T cells and preparation method and application of MUC16 targeted chimeric antigen receptor T cells
InactiveCN110527667APromote amplificationEfficient and specific killingPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenChimeric Antigen Receptor T-Cell Therapy
The invention provides a MUC16 targeted chimeric antigen receptor and MUC16 targeted chimeric antigen receptor T cells. The MUC16 targeted chimeric antigen receptor can exclusively target MUC16, a CD27 signal region is used as a costimulatory signal region, amplification of the T cells in bodies of patients is promoted, tumor cells can be efficiently and specifically killed and damaged, and the vitality and the lethality of the cells can be well maintained. The invention further provides a preparation method and application of the MUC16 targeted chimeric antigen receptor T cells.
Owner:SHENZHEN BINDEBIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com