HPK1-targeted gRNA and editing method of HPK1 gene
A targeting and gene technology, applied in the fields of molecular biology and immunology, can solve the problems of complex operation, enhanced killing activity of T cell hpk1 gene, and high technical requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Design and synthesis of embodiment 1 gRNA
[0108] 1. Design of Guide RNA
[0109] The gRNA loading plasmid is pUC57kan-T7-gRNA, the gRNA targeting HPK1 is designed, and the gRNA targeting PD1 is used as a control. The sequence of the gRNA specifically targeting HPK1 and the genome is GACCTGGTGGCACTGAAGA (located in the second exon of HPK1, SEQ ID NO: 1); the sequence of the gRNA specifically targeting PD1 paired with the genome is GGCCAGGATGGTTCTTAGGT (located in the first exon of PD1 exon, SEQ ID NO: 2).
[0110] HPK1 gRNA: F:5'-TAGG GACCTGGTGGCACTGAAGA-3' (SEQ ID NO:3)
[0111] R: 5'-AAAC TCTTCAGTGCCACCAGGTC-3' (SEQ ID NO: 4).
[0112] PD1gRNA: F: 5'-TAGG GGCCAGGATGGTTCTTAGGT-3' (SEQ ID NO: 5)
[0113] R: 5'-AAAC ACCTAAGAACCATCCTGGCC-3' (SEQ ID NO: 6).
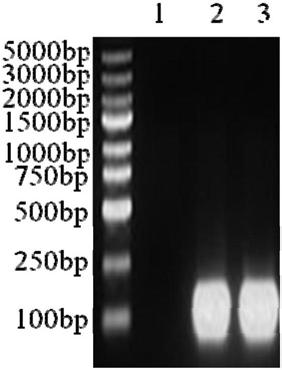
[0114] Synthesize the gRNA coding strand and complementary strand according to the above sequence, and insert the double-stranded DNA template formed by the annealing of the two DNA strands of HPK1gRNA and PD1gR...
Embodiment 2
[0117] Example 2 Recombinant expression and purification of Cas9 protein
[0118] The loading plasmid of Cas9 is PGEX4T-1, and the codon-optimized full-length human cas9cDNA is obtained by PCR. The template is the plasmid PUC19-T7-CAS9, and nuclear localization signals are added to the 5' and 3' ends of the Cas9 sequence (NLS) to promote the nuclear import of cas9 protein.
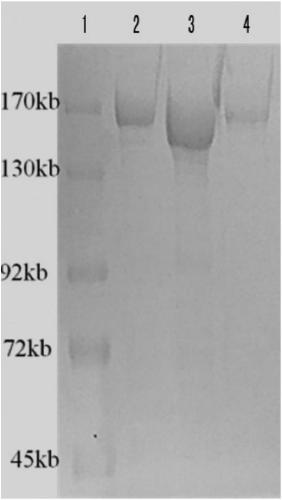
[0119] After the plasmid was constructed successfully, express the CAS9 protein with E.col, purify it through a GST column, concentrate and collect the protein, and cut off the GST tag with thrombin, an obvious single protein band can be seen around the 160kd band ( figure 2 ).
Embodiment 3
[0120] Example 3 In vitro expansion and transfection of human peripheral blood mononuclear cells
[0121] by using Extraction of human peripheral blood mononuclear cells by centrifugation and rapid separation of formazan sodium solution. CD3-positive peripheral blood mononuclear cells were sorted out with CD3 magnetic beads, and T cells were activated with CD3 / 28 antibody, with LONZA-X-VIVO 15 medium containing 100U / ml IL-2 at 37°C / 5% CO 2 The virus transfection and electroporation of the cells were carried out after culturing the cells under the condition of 2 days.
[0122] After cas9 protein and gRNA mRNA were mixed in proportion, they were left at room temperature for 10 minutes, while 4.0×10 6 CD3+ T cells (after CD3 / CD28 antibody activation in vitro for 48 hours) were transferred to a 15ml centrifuge tube, centrifuged at 500×g for 5 minutes, resuspended with 400ulopti-medium (containing cas9 and gRNA mixture), and transferred the cell mixture to the gap Place the ele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com