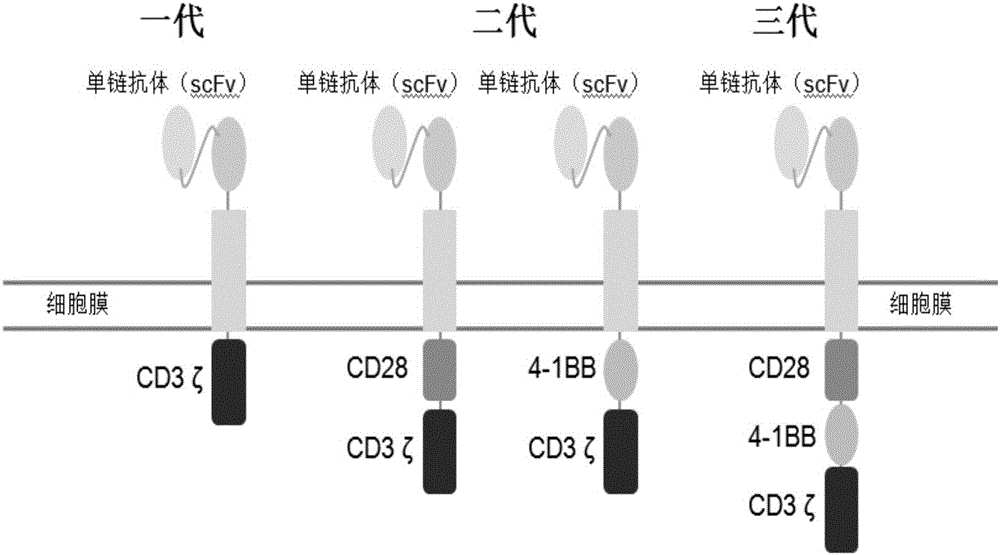
Chimeric antigen receptor T cells as well as preparation method and application thereof
A chimeric antigen receptor and cell technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of strict conditions for patient enrollment and patients who cannot be enrolled in group therapy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
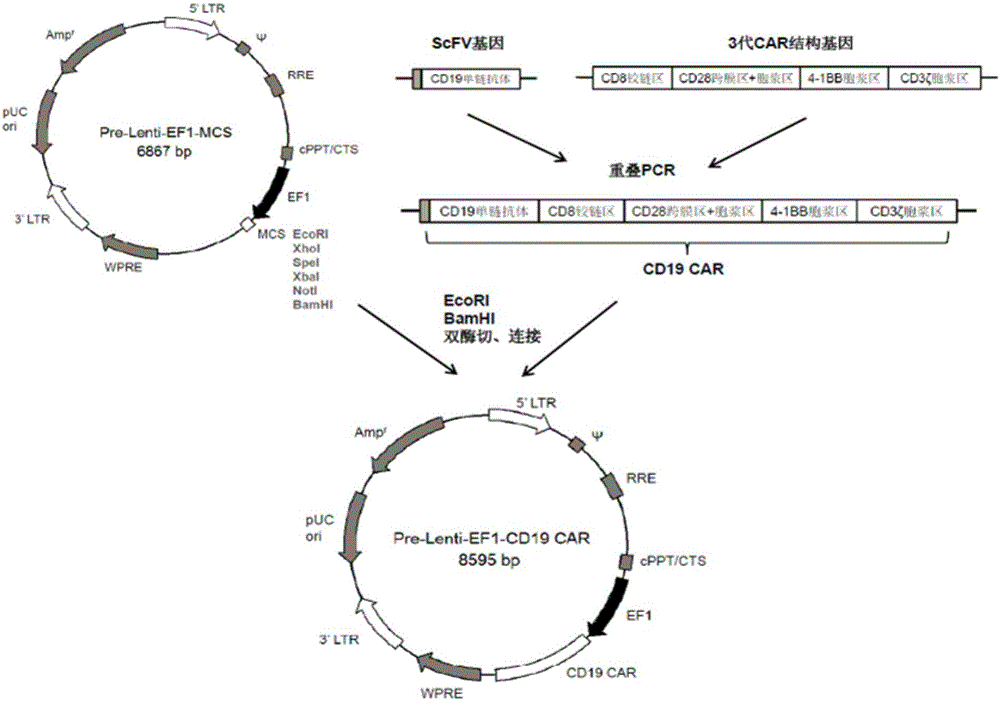
[0028] Example 1: Pre-Lenti-EF1-CD19CAR vector construction
[0029] 1. Synthetic ScFV gene: the heavy chain amino acid codon encoding CD8 signal peptide, sequence number CAA74659.1 was obtained by PCR method and optimized to nucleotide, (G4S) 3 The hinge region and the amino acid codon of the light chain with the sequence number CAA74660.1 are optimized as a nucleotide sequence of a nucleotide fragment of the ScFV gene of the CD19 single-chain antibody, the base sequence of which is shown in SEQ ID NO:1.
[0030] 2. Synthesize the third-generation CAR structural gene, and obtain the nucleosides of the CAR structural gene encoding the nucleotide fragments of the CD8 hinge region, CD28 transmembrane region, cytoplasmic region, 4-1BB cytoplasmic region and CD3ζ cytoplasmic region by PCR Acid fragment, its base sequence is shown in SEQID NO:2.
[0031] 3. The synthetic ScFV gene and the third-generation CAR structural gene were spliced by overlapping PCR to obtain the CD19CAR ...
Embodiment 2
[0066] Embodiment 2: cell killing experiment
[0067] 1. Lentiviral packaging
[0068] (1) 293T cells were cultured at 37°C, 5% CO 2 In the incubator, the medium is DMEM / 10% FBS.
[0069] (2) One day before packaging the virus, trypsinize 293T cells, 1×10 7 Cells / well were seeded in 10 cm Petri dishes.
[0070] (3) When transfecting cells, in addition to the Pre-Lenti-EF1-MCS-CD19CAR plasmid, each plasmid should be co-transfected with packaging plasmids psPAX2 and pMD2.0G. Among them, Pre-Lenti-EF1-MCS-CD19CAR uses 5 μg, psPAX2 uses 3.75 μg, and pMD2.0G uses 1.25 μg. For transfection, add the mixture of the above three plasmids to 500 μl opti-MEM medium, add 25 μl Lipofectamine 2000 reagent to 500 μl opti-MEM medium in another microcentrifuge tube, and then add the diluted transfection reagent dropwise On top of the diluted plasmid, mix well, centrifuge, and stand at room temperature for 20 minutes. Finally, add the mixture of plasmid and transfection reagent to a 10cm cu...
Embodiment 3
[0108] Embodiment 3: animal experiments
[0109] 1. Mouse strain: it is NSG immunodeficiency mouse strain (lack of T cell, B cell and NK cell function). 2. Tumor inoculation: using Raji lymphoma cell line, 1x10 6 Cells / 200ul PBS tail vein injection (6 mice).
[0110] 3. CART injection: 2 days after Raji tumor cell inoculation, Control or CD19CART cells (1x10 7 cells / 200ul PBS, a group of 3 mice), see Figure 6 . Depend on Figure 6 It can be seen that the control CART cannot effectively inhibit the growth of lymphoma cells in the liver (the white plaques are tumors formed by lymphoma cells), while CD19 CART can effectively inhibit lymphoma.
[0111] 4. Observe every 2 days, measure the body weight of the mice, until 2 weeks after the CART cell injection, the mice in the control group (Control) begin to appear hindlimb paralysis, and die one after another. Dissect and observe, record the time of death, and sort out the survival curve, see Figure 7 . Depend on Figure 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com