Drugs contg. as active ingredient midkine or inhibitors thereof
A technology of active ingredients and inhibitors, which is applied in the field of pharmaceuticals that control the function of neutrophils, and can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
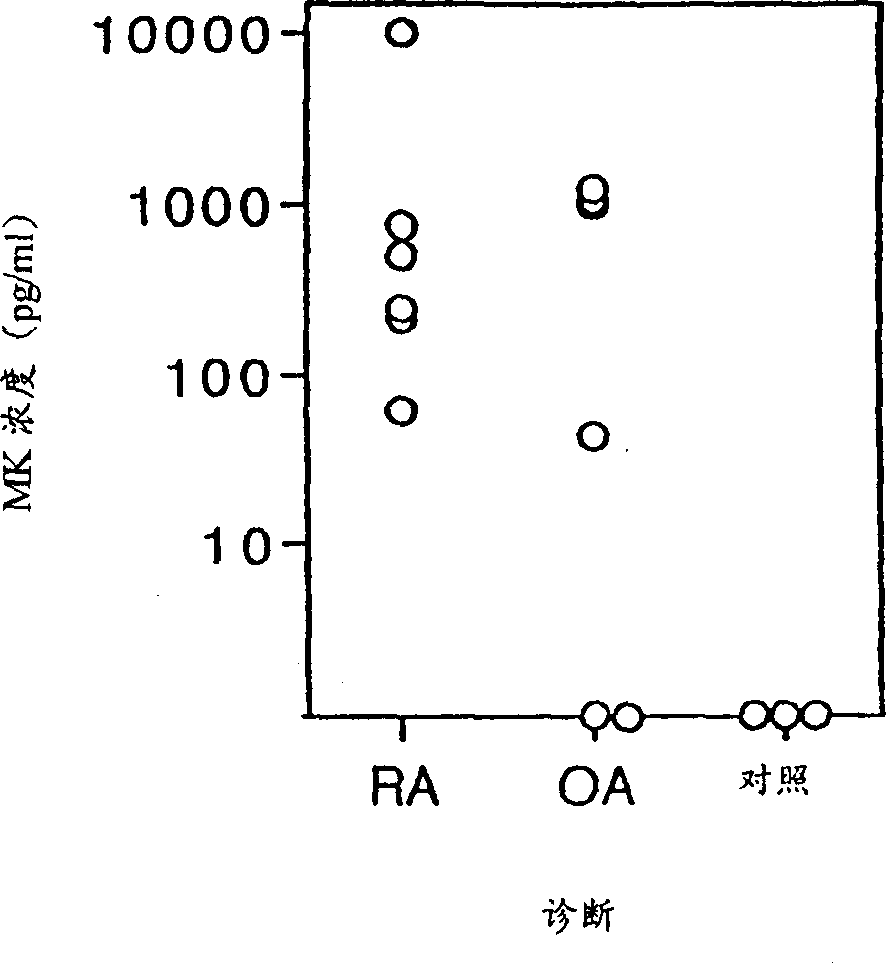
[0042] Detection of midkine(MK) by ELISA
[0043] Synovial fluid samples were collected by aspiration from patients with inflammatory synovitis in OA or RA (age range 26 to 72; mean age 52 years). MK was detected in synovial fluid samples by ELISA (Muramatsu, H et al., J. Biol. Chem., 119:1171-1175, 1996). MK was undetectable in any synovial fluid samples from 3 healthy subjects, but was detected in all samples from 6 RA patients ( figure 1 , MK concentration is 62 to 10,000 pg / ml). Significant amounts of MK were detectable in 4 of 6 samples each from a different OA patient ( figure 1 , below the detection limit, to 1225pg / ml). The results indicated that MK levels in synovial fluid were significantly correlated with the inflammatory state of synovitis.
Embodiment 2
[0045] Immunohistochemical detection of MK
[0046]Synovial tissues were obtained from whole knee sections of 3 RA patients and 2 OA patients. All biopsy samples contained proliferative inflammatory periosteal tissue, histologically characterized by proliferation of cells lining the synovium, extensive infiltration of lymphocytes and macrophages, and profuse vascularization. Immunohistochemical detection was performed using the method of Muramatsu et al. (Muramatsu, H et al., Developmental Biology, 159:392-402, 1993). Biopsy samples were fixed in neutral-buffered formalin, embedded in paraffin, and cut into 5 μm thick sections. Sections were incubated overnight at 4°C with anti-human MK antibody (15 mg / ml) in PBS containing 0.2% bovine serum albumin and 2% normal goat serum. Using rabbits immunized with chemically synthesized human MK purchased from Peptide Institute, anti-human MK antibody was prepared by the method of Muramatsu et al. Control samples were incubated with P...
Embodiment 3
[0049] Detection of MK by Western blot analysis
[0050] Synovial tissue extracts were subjected to Western blot analysis, samples were electrophoresed on SDS-polyacrylamide gels by the method of Laemmli (Laemmli, U.K., Nature 227:680-685, 1970), and samples were electrophoresed on SDS-polyacrylamide gels by Towin et al. Proc.Natl.Acad.Sci.USA, 76:4350-4354,1979) method to transfer the protein in the gel to the nitrocellulose membrane. Nitrocellulose membranes were incubated overnight at 4°C with Dulbecco's phosphate-buffered saline (PBS) containing 5% skim milk. Then it was incubated with diluted anti-human MK antibody (diluted to 20 mg / ml with 5% skim milk solution) for 2 hours at room temperature. The nitrocellulose membrane was washed with PBS containing 0.1% Tween 20, incubated with an affinity-purified anti-rabbit IgG-horseradish peroxidase conjugate (Jackson Immunoresearch Laboratories, Baltimore, USA), and incubated with 4-chloro - 1-naphthol staining.
[0051] High...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com