Application of midkine protein and medical device containing protein
一种医用装置、蛋白的技术,应用在用途及含该蛋白的医用装置领域,能够解决不能促使合成软骨基质、不能促进成软骨潜能的细胞软骨分化、不能促进软骨基质合成等问题,达到好增殖作用的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Preparation of recombinant protein rhMK
[0103] Refer to the literature "Zhang Z, Du L, Xiang D, Zhu S, et al. J Zhejiang UnivSci B, 2009, 10: 79-86" DNA sequence encoding human MK mature protein (GenBankNM_001012334) and pET30a+ (Novagen, USA) vector Ligated and transformed into E.coli (the strain Escherichia coli involved in the present invention has been described in "Zhang Z, Du L, Xiang D, Zhu S, et al. J Zhejiang Univ Sci B, 2009, 10: 79-86" public). Recombinant rhMK protein was purified by ion exchange chromatography. The purified protein is detected by reverse high-performance liquid chromatography, and its purity is greater than 98%. The endotoxin content in the recombinant rhMK protein was lower than 0.3EU / μg protein. The biological activity of the recombinant rhMK protein was detected by stimulating the growth of NIH3T3 cells.
Embodiment 2
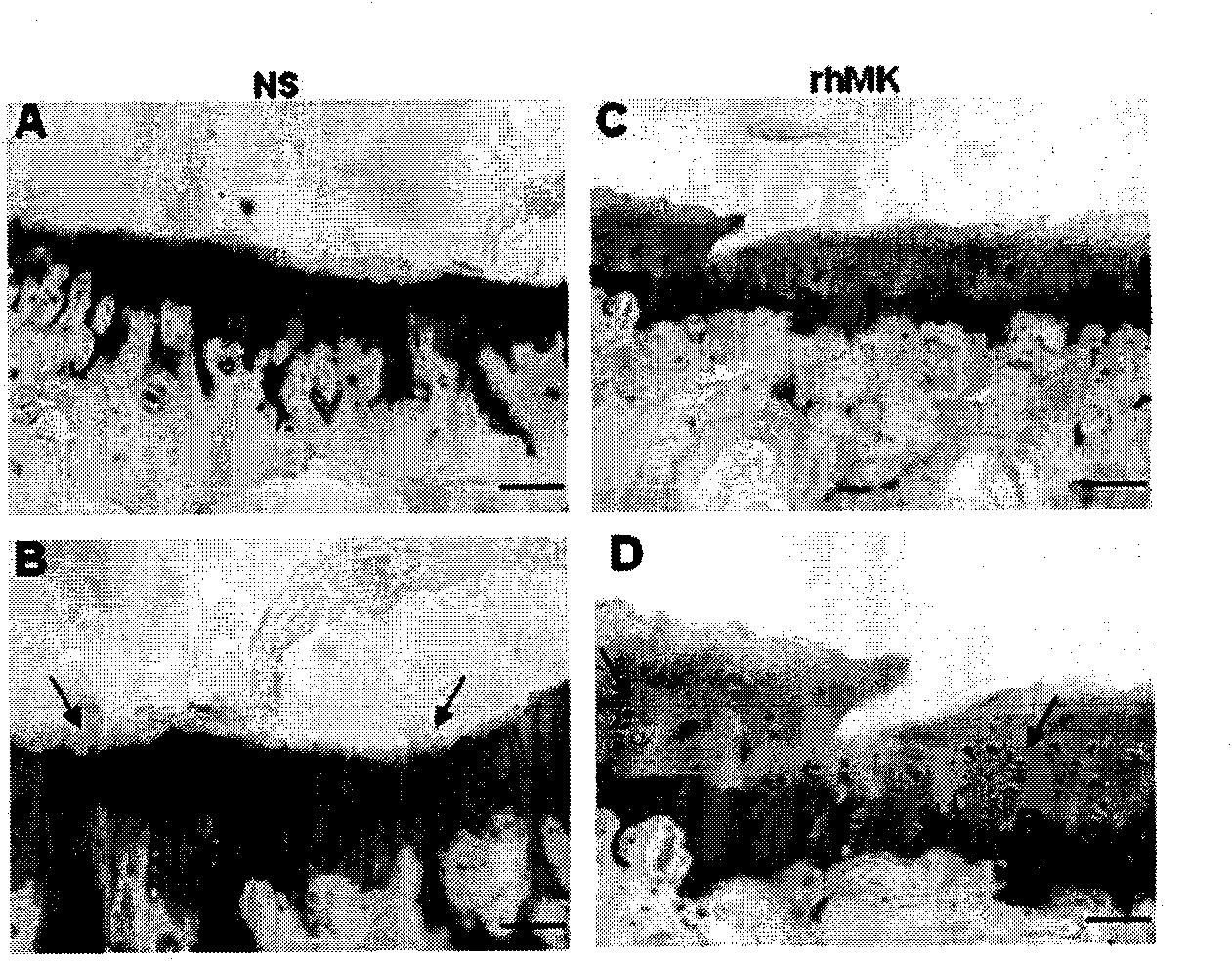
[0105] Effects of Recombinant Protein rhMK on Cartilage of Normal Mice, Rats and Rabbits
[0106] step one
[0107] All animals were administered by subcutaneous injection, and the injection volumes of the recombinant protein group and normal saline (NS group) were both 0.5 ml, and the rhMK used was diluted to the required concentration with normal saline for injection. Eight-week-old male C57BL / 6J mice were divided into six groups for injection: NS group, rhMK group (10, 33, 100, 300, 900 μg / kg body weight) for one week, with 3 mice in each group. Three-week-old SD rats were divided into two groups: NS group and rhMK group (injected with 175 μg / kg body weight of recombinant protein), continuously injected for one week, with 3 rats in each group. Two-month-old New Zealand white rabbits were divided into two groups: NS group and rhMK group (injected with 92.5 μg / kg body weight of recombinant protein), injected continuously for two weeks, with 3 rabbits in each group.
[0108]...
Embodiment 3
[0122] Establishment of full-thickness defect model of articular cartilage in rabbits and repair of the defect with rhMK
[0123] Step 1: 2-month-old male New Zealand white rabbits, weighing 2.0-2.5 kg, were anesthetized with 3% sodium pentobarbital (30 mg / kg body weight) through ear veins, and the knee joints on both sides of the rabbits were skinned. The surgical area was routinely disinfected. Under aseptic conditions, an arc-shaped incision was made on the medial side of the knee to expose the femoral condyle surface. A full-thickness cartilage defect with a diameter of 3 mm and a depth of 4 mm was drilled with an electric drill, and then the wound was closed layer by layer with 4-0 suture . After the operation, each rabbit was injected with 400,000 units of penicillin every day for one week to prevent wound site infection. 24 hours after the operation, normal saline and recombinant protein rhMK were injected into the joint cavity. The defective knee joint on one side of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com