Immunogenic peptides derived from the midkine protein, as an anticancer vaccine
A midkine and protein technology, applied in animal/human proteins, cancer antigen components, vertebrate antigen components, etc., can solve the problem that the immunogenicity of midkine protein has not been studied.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Example 1 : CD8 specific for midkine protein peptide + Induction of T response
[0160] Materials and Methods
[0161] a) peptide
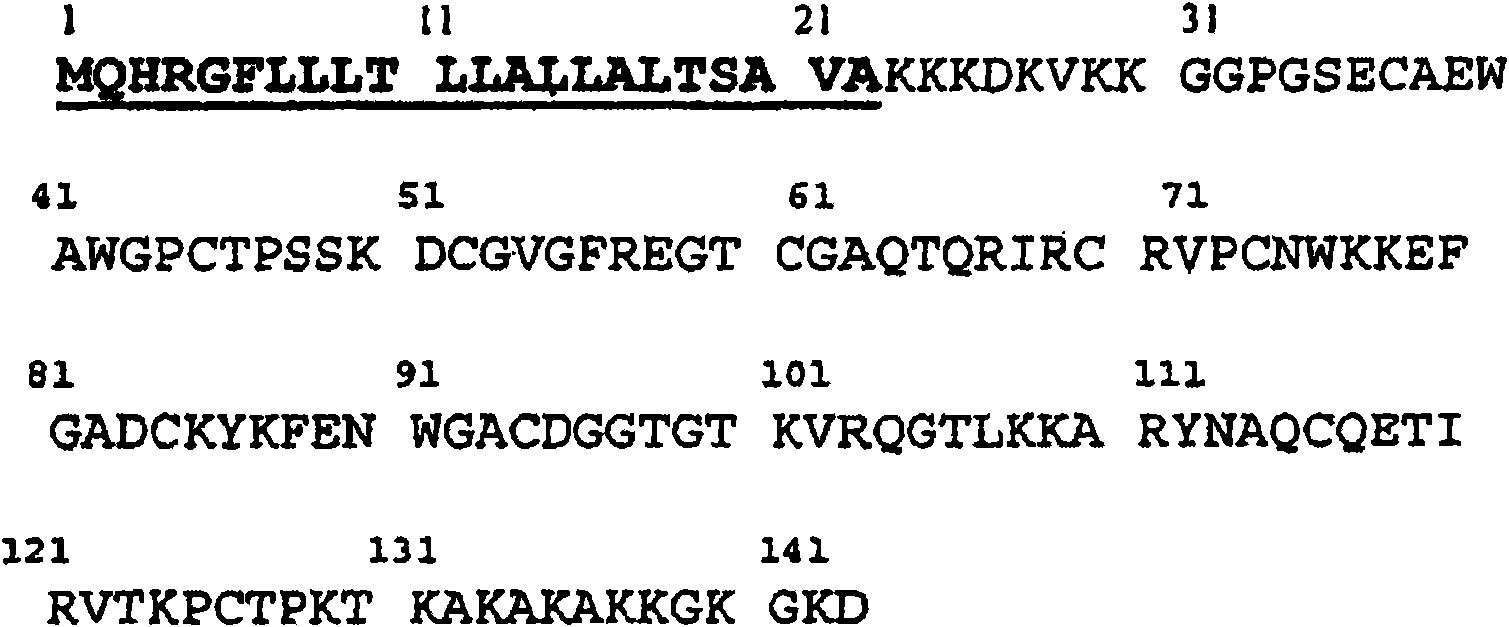
[0162] Seven peptides represent potential CD8 restricted by HLA-A2 molecules + T epitopes, which are the most widely represented class I HLA alleles in the Caucasian population, were identified by using the BIMAS program ( http: / / www- bimas.cit.nih.gov ) is selected. Sequences of selected peptides are shown in Table IV and the accompanying Sequence Listing.
[0163] Table IV : list of selected peptides
[0164] peptide
sequence
SEQ ID NO:
MDK13-21
ALLALTSAV
4
MDK12-21
LALLALTSAV
3
MDK14-22
LLALTSAVA
6
MDK13-22
ALLALTSAVA
5
MDK114-122
AQCQETIRV
8
MDK113-122
NAQCQETIRV
7
MDK63-72
AQTQRIRCRV
17
[0165] These peptides were synthesized in solid phase parallel synthesis according to the Fmoc strat...
Embodiment 2
[0186] Example 2 : Detection of CD8 specific for midkine peptides by labeling with specific tetramers + T lymphocytes
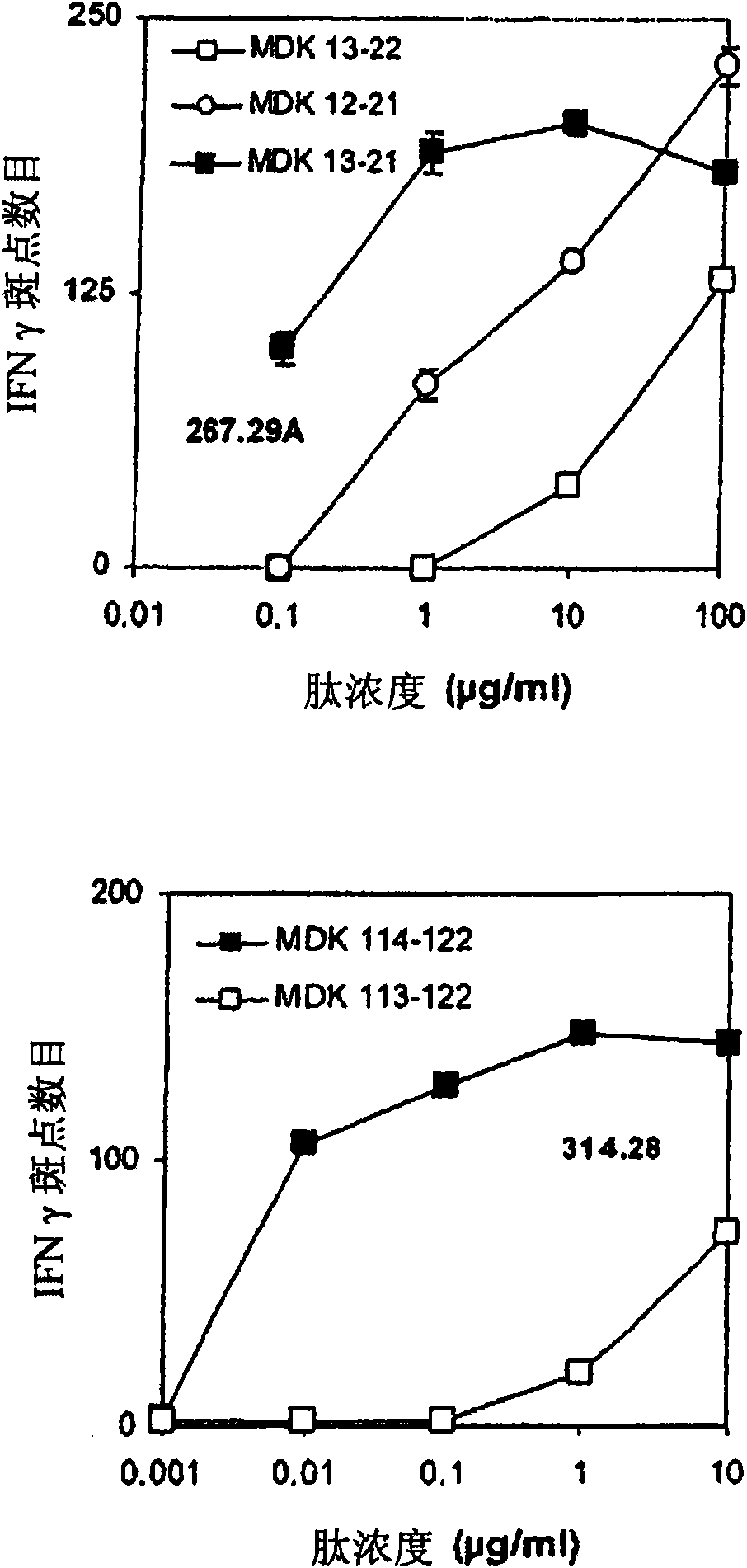
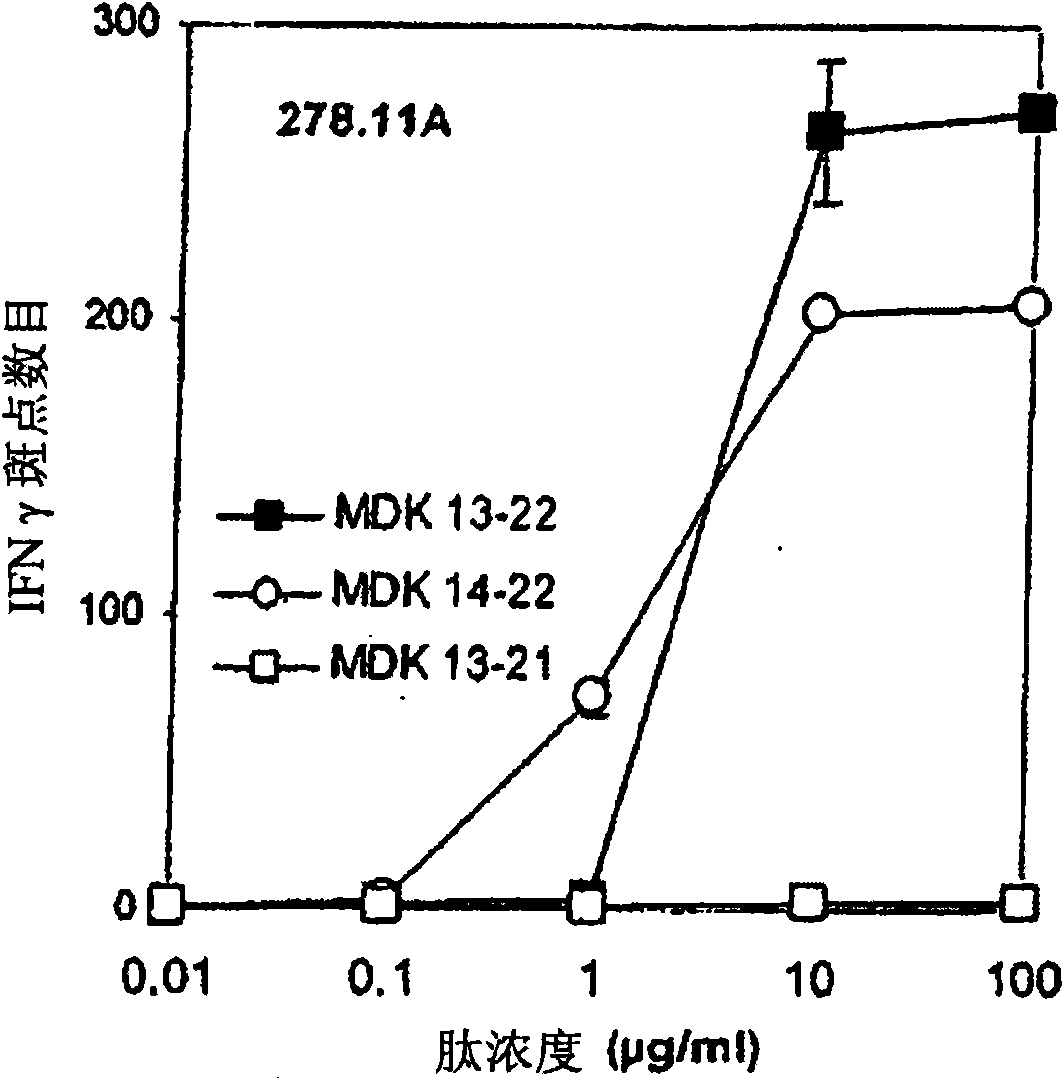
[0187] Each lymphoid cell line (500000 cells) obtained in Example 1 was labeled with tetramer at 50 μg / ml in 200 μl PBS / 2% FCS in the dark at 4°C. These tetramers are biotinylated HLA-A2 molecules loaded with peptides 13-21 or 114-122 and complexed with phycoerythrin-tagged streptavidin, and according to Novak et al. (J.Clin.Investig ., 1999, 104, R63-R67) or the technique described in Kuroda et al. (J. Virol., 2000, 74, 18, 8751-8756). Cells were then washed twice in PBS before labeling with FITC anti-CD8 antibody (BD Biosciences) for 30 minutes at 4°C. After washing in PBS, cells were fixed with 50 [mu]l of PBS containing 1% paraformaldehyde (PAF). These markers were analyzed on a FACSCalibur flow cytometer (BD Biosciences). The results are shown in Figure 7 middle.
Embodiment 3
[0188] Example 3 : Induction of CD4 specific for peptides of the midkine protein + T-reaction
[0189] 1) Materials and methods
[0190] a) peptide
[0191] Coverage of human midkine (SwissProt P21741, SEQ ID NO: 2 and figure 1 ) peptides of 15 amino acids (15-mers) of the full sequence, selected based on the presence of aromatic or hydrophobic residues at positions 3 or 4, for use in the P1 pocket of HLA-DR and HLA-DP4 molecules mid anchor.
[0192] Selected peptide sequences are shown in Table V and the accompanying Sequence Listing.
[0193] Peptides were synthesized in solid-phase parallel synthesis according to the Fmoc strategy, purified by HPLC, and verified by mass spectrometry (ES-MS).
[0194] Table V : Selected peptides (SEQ ID NO: 9, 10, 13-15 and 18-30)
[0195]
[0196]
[0197] *Refers to the sequence of the 143 amino acid human midkine precursor (SwissProt P21741, figure 1 and SEQ ID NO: 2) to number the positions.
[0198] b) HLA II / pepti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com