Enzyme-mediated modification of fibrin for tissue engineering: fibrin formulations with peptides
a technology of fibrin and peptides, which is applied in the direction of peptide/protein ingredients, cell culture active agents, unknown materials, etc., can solve the problems of not very much research on the concentration dependent effect of fibrin in a three-dimensional matrix, and the maximum migration rate is not high, so as to achieve enhanced and enhanced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
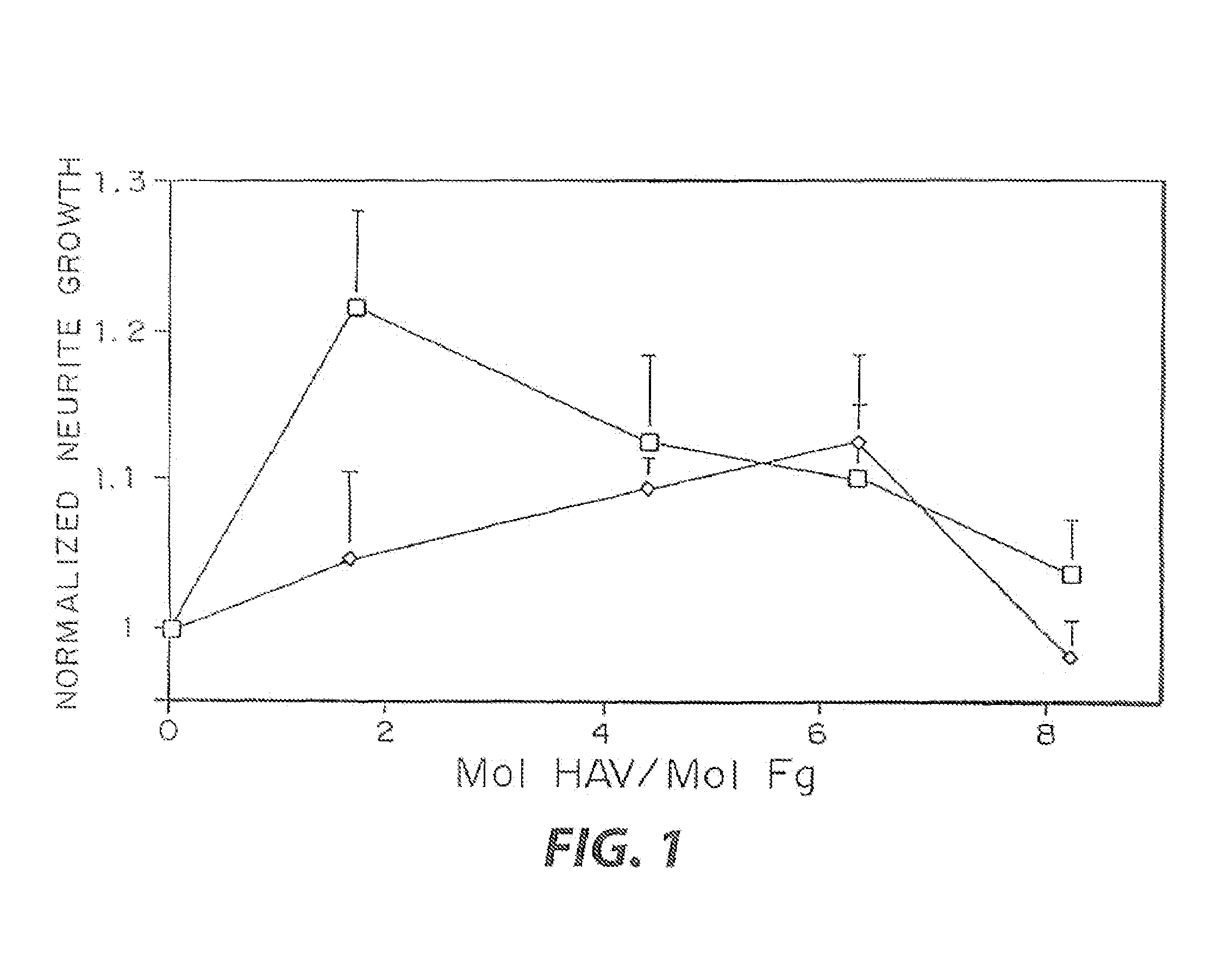
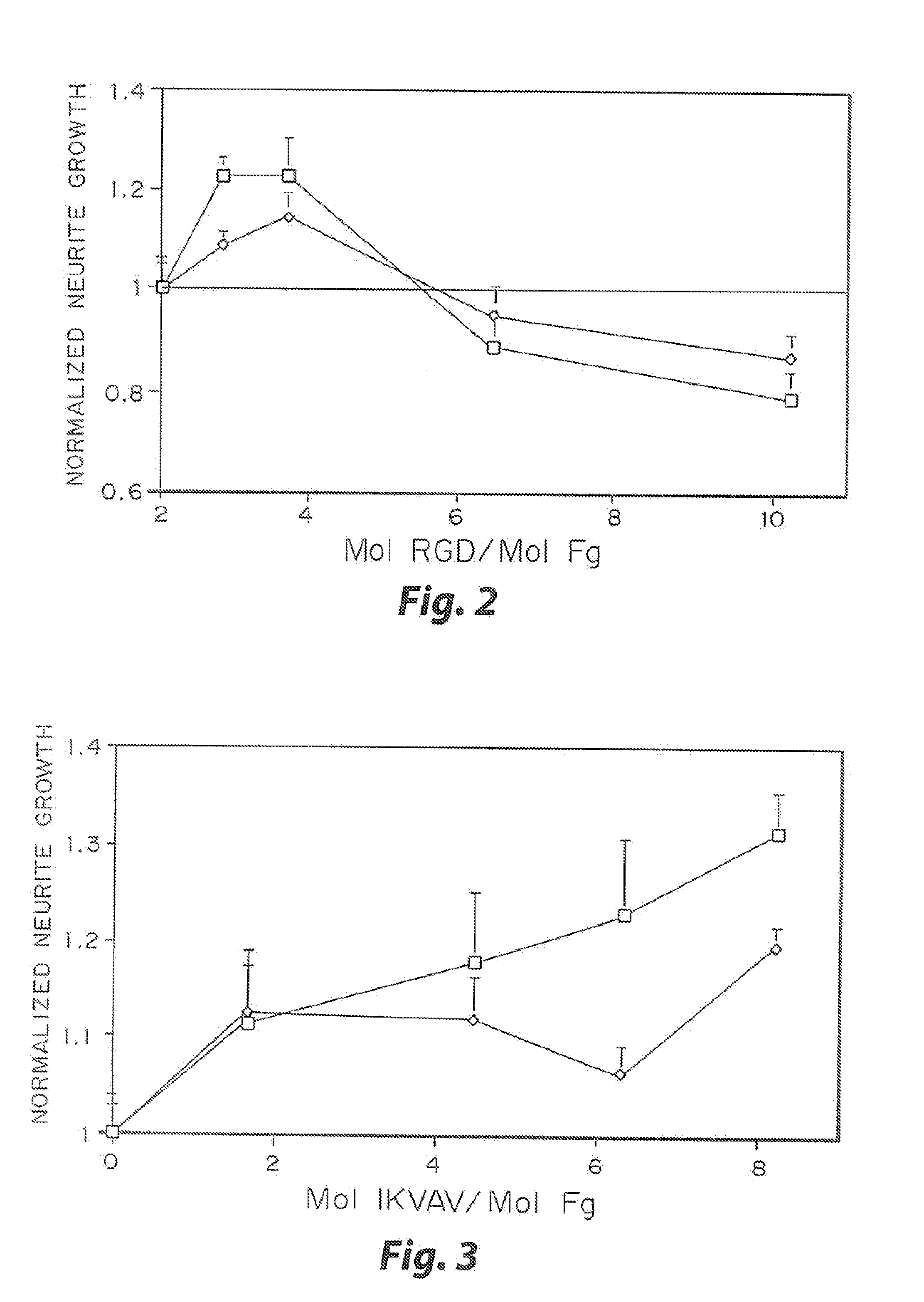
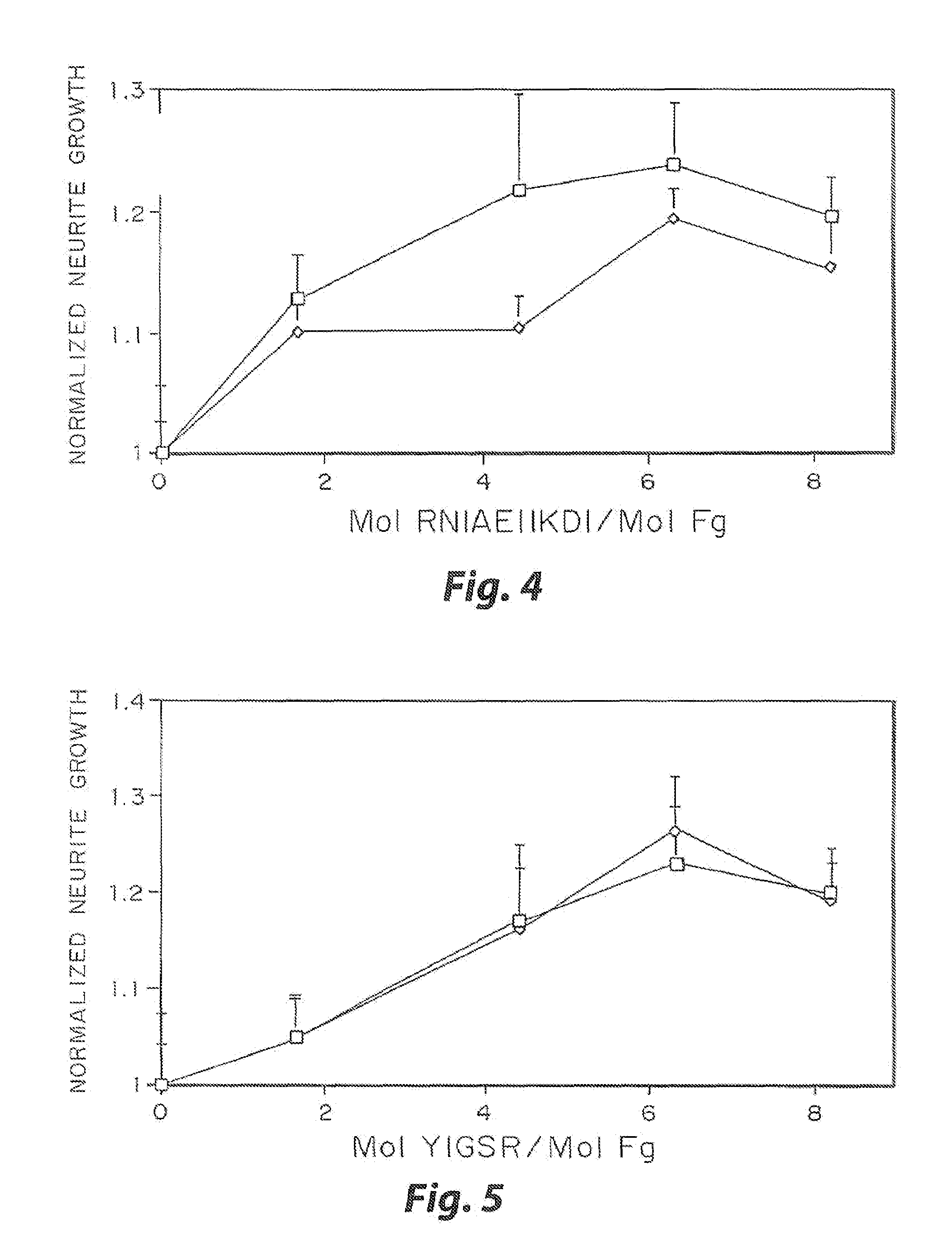
[0032]The biological effect of incorporated peptides in a three dimensional matrices demonstrated in the present example. Multiple peptide sequences from extracellular matrix proteins have been chosen. These proteins were chosen in part because of their reported ability to enhance neurite outgrowth (Yamada, 1991, Tashiro, et al., 1989). These proteins have been tested at various concentrations. To test these peptides, specific selected peptide sequences were cross-linked into a three dimensional fibrin matrix with a day 8 dorsal root ganglia embedded into the gels. The neurites were grown for 48 hr, and the migration rate of the neurties extending from the ganglia was quantified for each condition at both 24 and 48 hr. This growth was then normalized to the growth in unmodified fibrin. The ability for some of these peptides to enhance neurite outgrowth was found to increase with peptide concentration, while other peptides reach a maximal enhancement at a moderate peptide concentrati...
example 2
Fibrin Gels With Multiple Peptides
[0033]Because two of the peptides that were tested were found to have maximal effect on the neuronal cell model employed at low concentrations, it is possible to incorporate these peptides at a low concentration and still observe a large neuronal effect, leaving many cross-linking sites open. The remaining sites can then be occupied with a different peptide which has it's maximal effect at a high concentration.
[0034]The present inventors employed the above approach using with several peptides. In one example, HAV (SEQ ID NO:6) was cross-linked at 2 mol / mol fibrinogen in combination with the following peptides at 6 mol / mol fibrinogen: IKVAV (SEQ ID NO:1), RNIAEIIKDI (SEQ ID NO:5), YIGSR (SEQ ID NO:3) and DGEA (SEQ ID NO:4). The growth obtained with the peptides grafted together, with the peptide grafted alone and the theoretical sum derived from the results of the two peptides grafted separately is shown in FIG. 6. The cross-linking of HAV (SEQ ID NO...
example 3
Custom-Designed Gel Matrix for Neurite Growth
[0041]There are four components necessary for creating a cross-linked fibrin gel; fibrinogen, calcium, thrombin and factor XIIIa, and the structural characteristics of the material. These four components can be modified by changing the concentration of any one of them. There are two main characteristics that determine the structure of the fibrin; the density of the fibrin bundles and the thickness of each individual bundle. These two properties will then control the ability of cells to infiltrate the matrix.
[0042]Increasing fibrin concentration from 5-15 mg / mL in the precursor mixture was found to result in fibrin gels with smaller fibrin bundles that are much denser. This resulting material has been shown to be more difficult for neurties to migrate through. When the calcium concentration was increased from 2-10 mM, the fibrin bundles got thicker, but the spacing between these bundles became greater. Changing the fibrin density clearly c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Substance count | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com