Therapeutic angiogenic factors and methods for their use
A technology of angiogenesis and angiogenesis, which is applied in gene therapy, cardiovascular system diseases, medical preparations containing active ingredients, etc., and can solve problems such as the loss of polypeptide growth factor gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
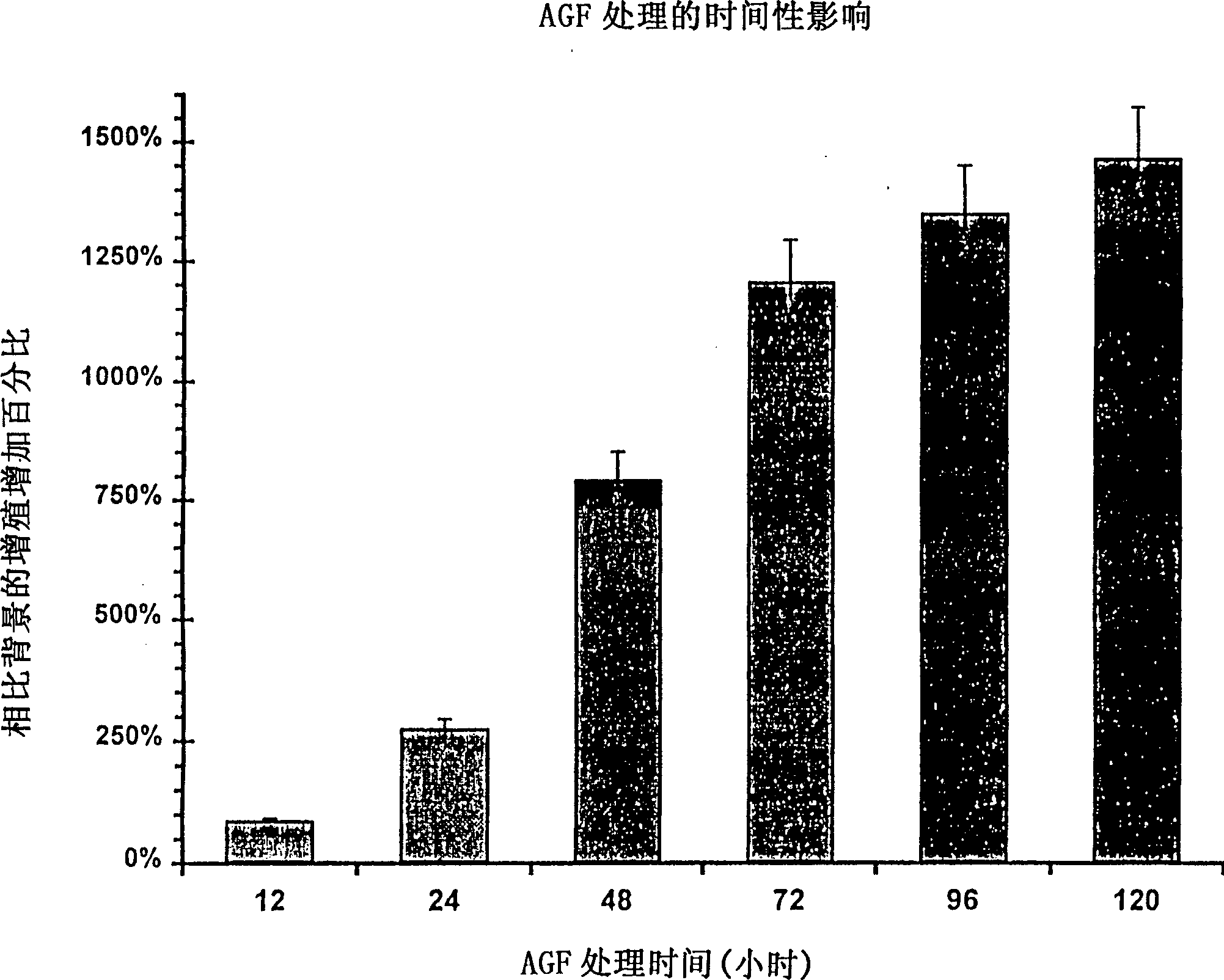
[0093] Example 1: Use of angiogenic factors in vitro
[0094] Recombinant human pleiotropin (PTN) was isolated as described by Fang et al., J. Biol. Chem., 267:25889-25897 (1992). To determine the percentage increase in proliferation of endothelial cells following in vitro stimulation with PTN, endothelial cells (HUVEC, human umbilical vein endothelial cells, American Type Culture Collection, #CRL-1730) were cultured at 10 4 Cells / well were seeded in 2 ml of F12K medium (Life Technologies (Rockville MD), #11765054 and #16140071, respectively) containing 10% fetal calf serum in 12-well tissue culture plates. After about 6 hours, the cells were attached to the culture plate, and 50 ng of PTN dissolved in 50 μl of PBS buffer (phosphate-buffered saline) was added to each well to be treated (6 treatment groups, each group n=6). An equal volume of PBS alone was added to each control well (n=6) to determine background proliferation levels. At each 24-hour time point, medium was rem...
Embodiment 2
[0095] Example 2: In vivo treatment of mouse wounds with angiogenic factors
[0096] PTN was isolated as described in Example 1. To determine the effect of topical PTN treatment in vivo on the subcutaneous vasculature of mice, matrix was injected bilaterally under the loose rib skin of 5 (n=10) BALB / c mice per group (Harlan Sprague-Dawley, Indianapolis, IN) implant. To prepare the implant, PTN protein dissolved in PBS solution (above) is mixed with Matrigel, which is liquid at room temperature TM (Collaborative Research, MA) to a concentration of 10 μg / ml. Control implants were prepared in a similar manner, but without PTN in the buffer. Since the matrix solution turns into a gel when the temperature rises above room temperature but below body temperature of 37°C, the matrix solution is injected into the subcutaneous pouch using a 16-gauge needle at 1 ml per site. At body temperature, the gel solution becomes a partially cured matrix. At each time point, the corresponding g...
Embodiment 3
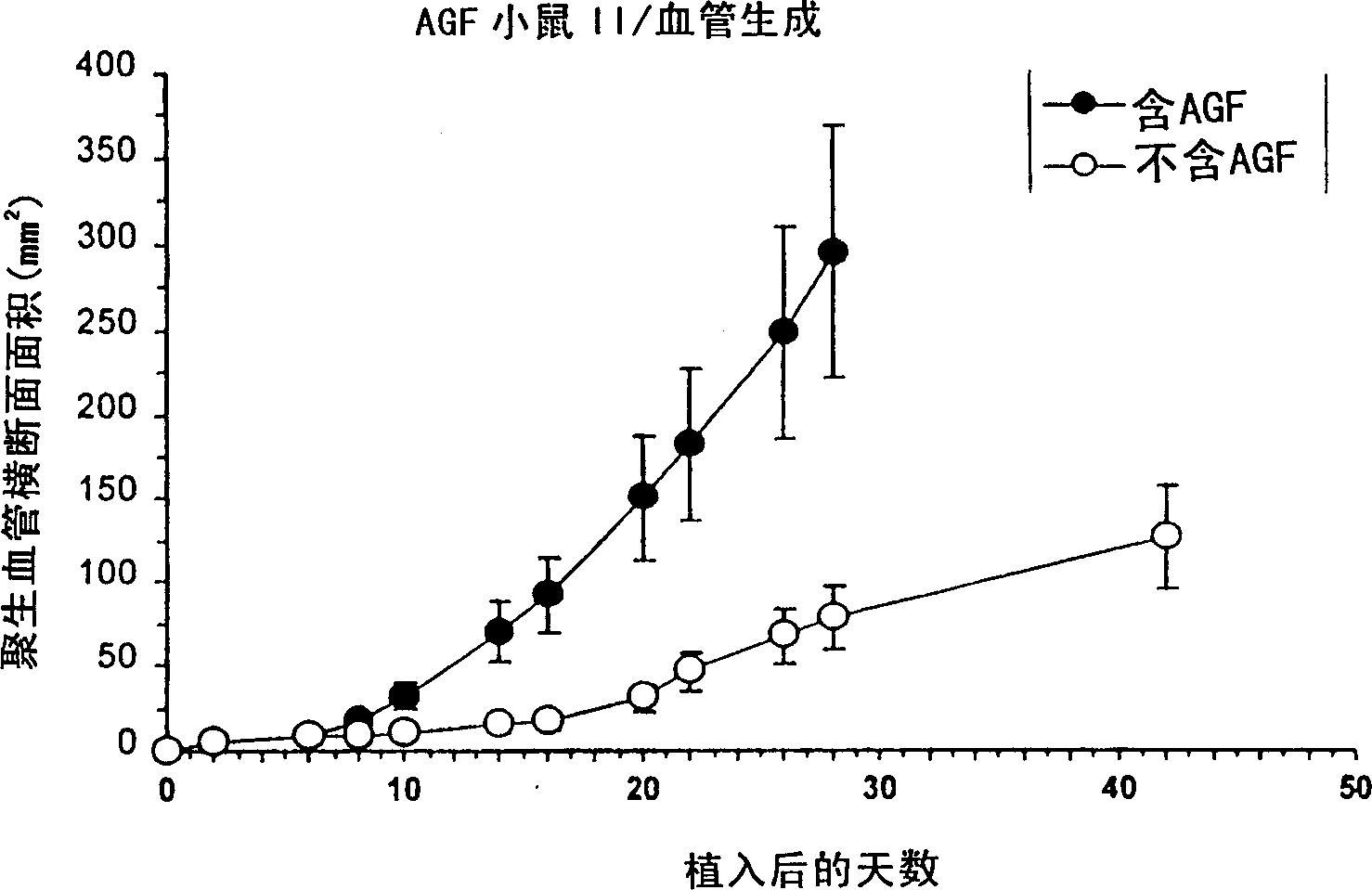
[0097] Example 3: Angiogenesis in vivo using a controlled delivery matrix
[0098] PTN was obtained as described in Example 1. To determine the effect of continuous topical PTN treatment on the functional vasculature in vivo, the well known Folkman CAM (chicken chorioallantoic membrane) assay was used. After fertilization 5 days' eggs (local Leghorn white, Half Moon Bay, CA) egg shell is partly opened, Vasotrophin TM The system (Angiogenix Corporation, Burlingame, CA) was placed at the leading edge of a CAM approximately 15 mm in diameter. Vasotrophin used TM The system is a 500 μl bioerodible pellet consisting of 1 μg / ml PTN formulated into a poly(lactide-co-glycolide) matrix (PLGA, Absorbable Polymer Technologies, Birmingham, AL), or each containing 500ng PTN. A control pellet was generated in a similar manner, but without PTN. Over the next two weeks, the CAMs were observed with the naked eye, and differences in vessel growth patterns were observed, visualized by a dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com