Anticoagulant fusion protein anchored to cell membrane
a fusion protein and anticoagulant technology, applied in the field of inhibition of blood coagulation, can solve the problems of lysis and death of ecs of transplants, the inability to match ever-rising demands for xenografts, and the inability to supply suitable transplant organs, so as to increase the effect of inhibiting the coagulation cascad
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
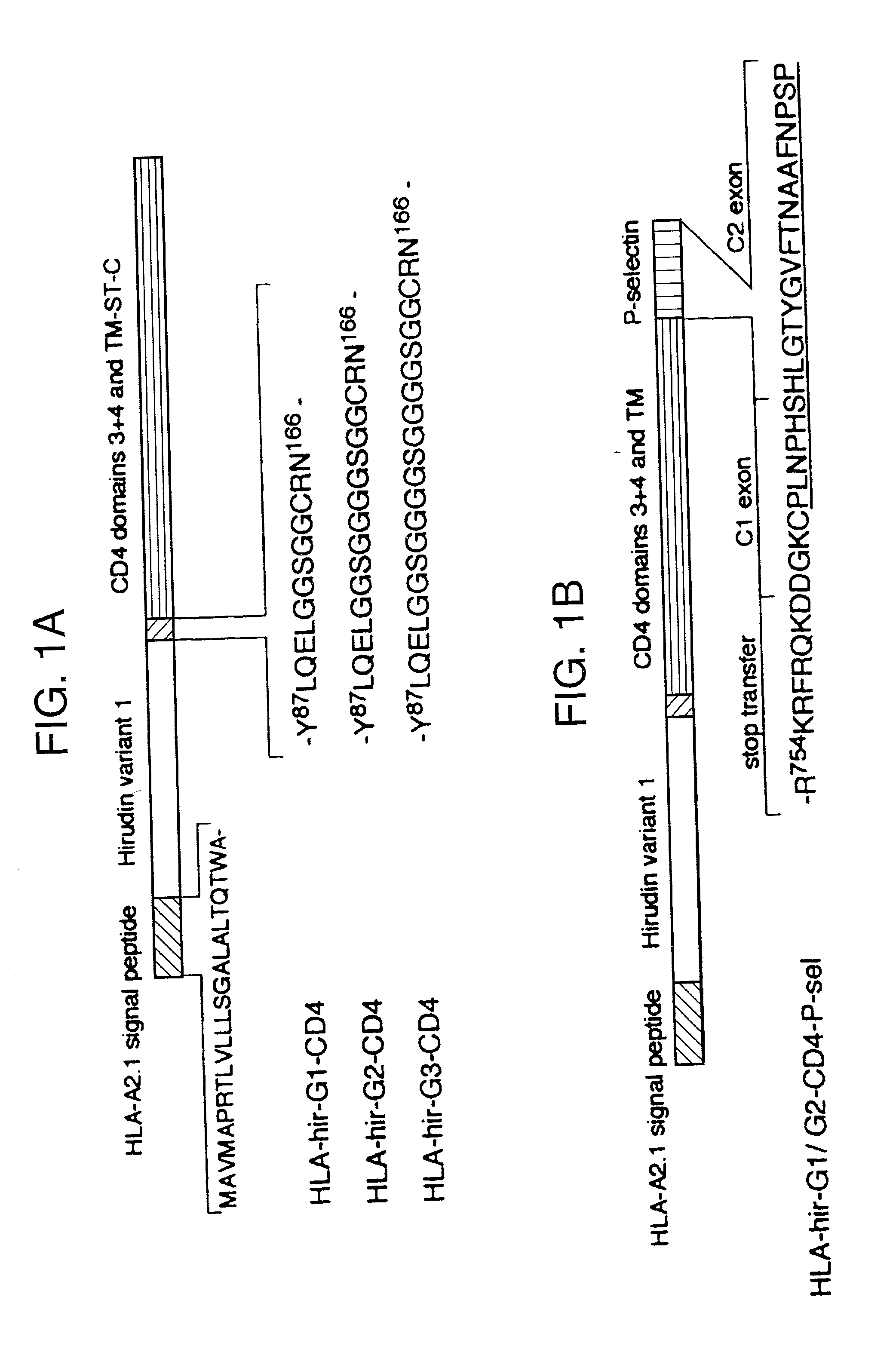
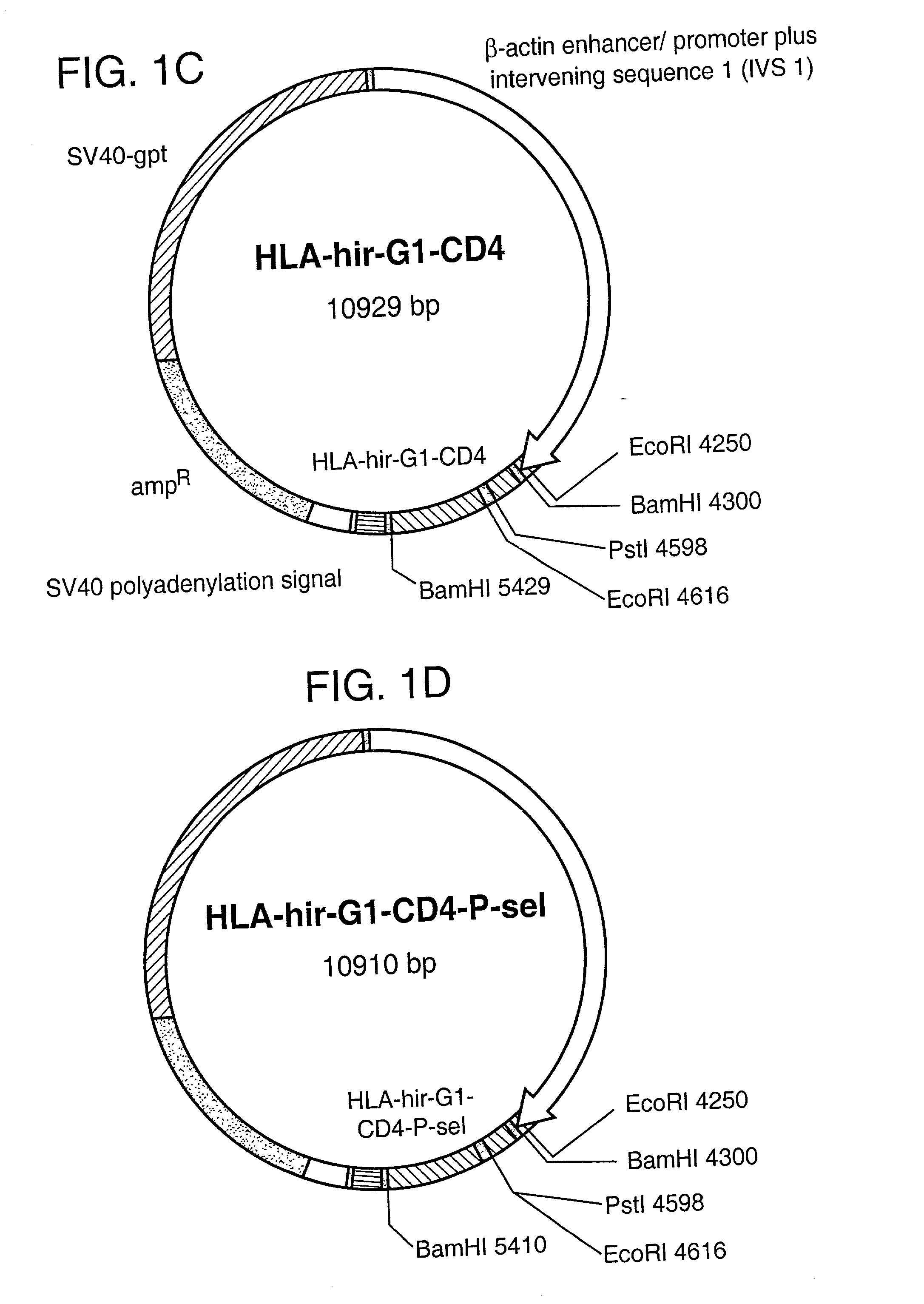
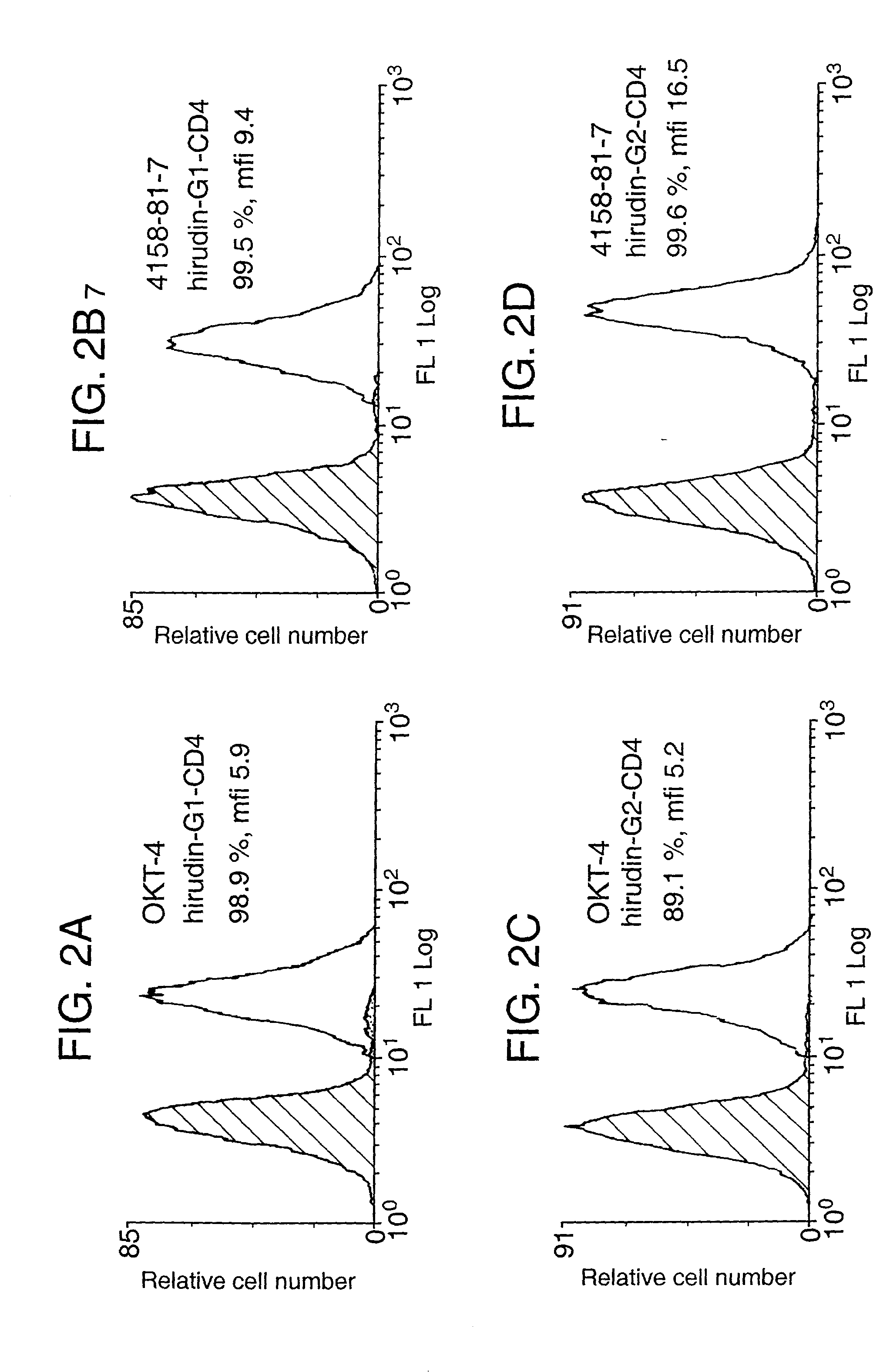
[0023] According to a first aspect of the present invention there is provided a protein comprising a region with anticoagulant activity and a region which can anchor said protein to a cell membrane. Preferably this is a chimeric protein, that is to say the anchor region and anticoagulant region are derived from different proteins.
[0024] The anticoagulant region can comprise the sequence of any anticoagulant polypeptide. Examples of such anticoagulant polypeptides include heparin, TAPs, antithrombin, hirudins, and TFPIs, along with their functional derivatives, such as fragments and derivatives which retain anticoagulant activity. Anticoagulant derivatives of thrombin, normally a procoagulant, have also been reported (Dang, 1997).
[0025] Preferably the anticoagulant region comprises the sequence of a hirudin. Hirudins include hirudin, hirudin derivatives, analogs (“hirulogs”), and variants (eg. hirudisins). For instance, it has been reported that sulphation at Tyr-64 increases the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| clotting time | aaaaa | aaaaa |
| clotting time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com