Preparation method and application of oxidized low-molecular-weight heparin-antithrombin compounds
A low-molecular-weight heparin and antithrombin technology, applied in the preparation of oxidized low-molecular-weight heparin-antithrombin complex-coated extracorporeal circulation pipelines, in the field of blood and biocompatible coatings, which can solve problems that cannot be completely prevented. Blood activation, plasma protein adsorption is not selective, etc., to achieve the effect of increasing affinity, reducing adsorption, and reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] According to the above technical principles, the present invention provides a method for preparing an oxidized low molecular weight heparin-antithrombin complex, the method comprising the following steps:
[0018] (1) React excess sodium periodate and low-molecular-weight heparin sodium injection for 30 minutes at room temperature in the dark, and centrifuge three times with an AmiconUltra-4 centrifugal ultrafiltration device with a molecular weight cut-off of 3K to obtain low-molecular-weight heparin injection. Molecular weight heparin sodium solution, wherein the average molecular weight of the low molecular weight heparin sodium is 5000.
[0019] (2) Mix the oxidized low molecular weight heparin sodium solution obtained in step (1) with antithrombin in 0.02mol / L pH7.3 phosphate buffered saline for 40 ° C water bath reaction for 14 days, promote the non-enzymatic glycosylation reaction between the terminal aldose residue of oxidized low molecular weight heparin and th...
Embodiment
[0024] Example: Preparation of polyvinyl chloride pipe coated with oxidized low molecular weight heparin-antithrombin complex and its physical and chemical properties detection
[0025] Add 2g of sodium periodate and 8ml of low molecular weight heparin sodium injection into a 25ml volumetric flask, then add deionized water to 25ml, and react in the dark for 30 minutes at room temperature; Centrifuge at 6500 rpm for 20 min at room temperature, discard the ultrafiltrate, add deionized water to the retentate, and repeat the centrifugation 3 times to obtain about 2 ml of oxidized low molecular weight heparin sodium solution.
[0026] 2mg antithrombin, 0.18gNaCl, 0.44ml0.2mol / LNaH 2 PO 4 Solution, 1.56ml0.2mol / LNa 2 HPO 4 solution, oxidized low molecular weight heparin sodium solution and 16ml deionized water were fully mixed to obtain a mixed reaction solution of about 20ml, which was put into 40 ° Reaction in C water bath for 14d; Add 2ml0.5mol / L sodium cyanoborohydride sol...
Embodiment 2
[0037] Example 2: Biocompatibility Evaluation of Polyvinyl Chloride Tubing Coated with Oxidized Low Molecular Weight Heparin-Antithrombin Complex
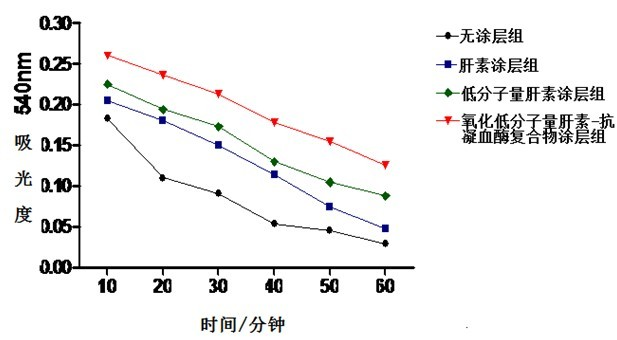
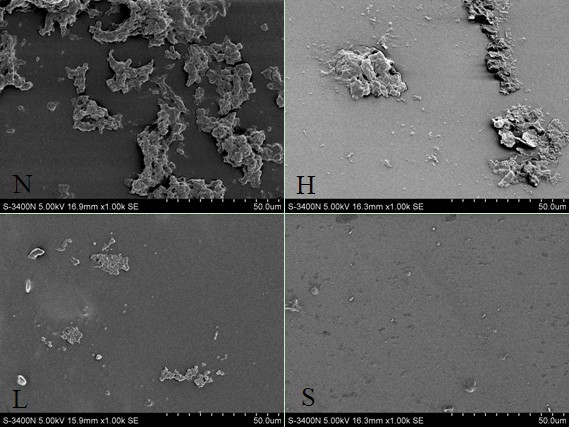
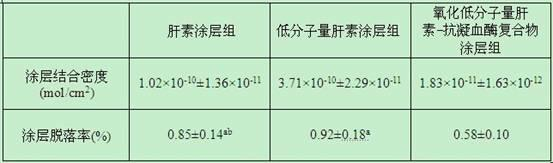
[0038] Biocompatibility evaluation experiments were carried out with the heparin-coated, low-molecular-weight heparin-coated and oxidized low-molecular-weight heparin-antithrombin complex-coated polyvinyl chloride pipes prepared in Example 1. The experiment was divided into five groups: control group, no coating group, heparin coating group, low molecular weight heparin coating group and oxidized low molecular weight heparin-antithrombin complex coating group. After human anticoagulated whole blood was simulated in vitro with Chandlar-Loop model for 4 hours, platelet count, fibrinogen content, plasma interleukin-8 (IL-8) and monocyte chemoattractant protein- 1 (MCP-1), and observe the inner surface of each group of pipelines with a scanning electron microscope. The results showed that the platelet counts in each group (×10 9 / L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com