Method for preparing human antithrombin-III product
A technology of antithrombin and AT-III, which is applied in the field of separation and purification of human antithrombin-Ⅲ, which can solve the problems of limitation and low recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
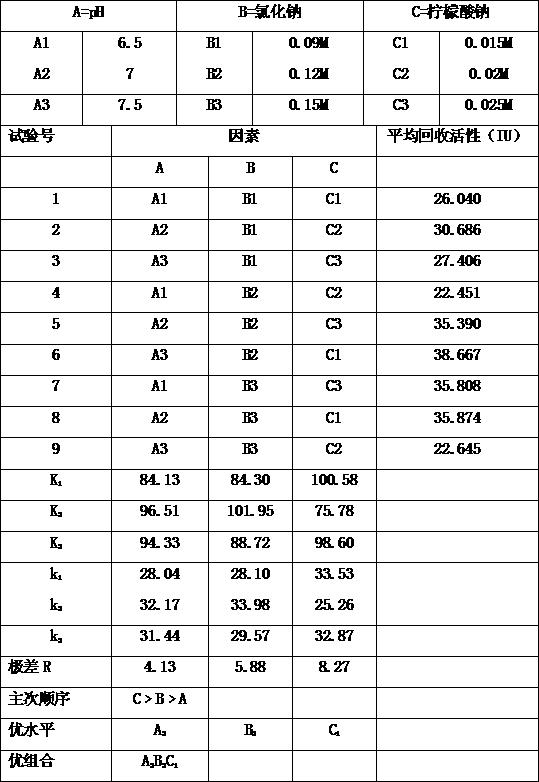
[0039] The loading chromatography conditions were optimized, and the pH value, sodium chloride concentration, and sodium citrate concentration of the component IV precipitation and extraction buffer were used as factors to design a three-factor three-level orthogonal experiment. The specific design scheme is shown in the table below . The average recovery activity of the three experiments was used as the investigation index, and the purpose of the experiment was to obtain the best combination of loading chromatographic conditions. The specific implementation process is as follows:
[0040] Weigh 50g of component IV precipitate and dissolve in different concentrations of extraction buffer, the ratio of extraction buffer to component IV is 4:1 (v / m) , adjust the pH value according to the design scheme, stir at room temperature for 1 hour, and then centrifuge it at 4°C and 4500 rpm for 30 minutes to remove the precipitate and collect the supernatant. Filter the supernatant, us...
Embodiment 2
[0044] 1. Basic requirements:
[0045] Debug the required equipment in the company's pilot workshop and mark it as a standby state;
[0046] Clean the required container pipelines and pilot plant and mark them as standby;
[0047] Prepare 100kg of component IV precipitate and store it in a -30°C freezer for future use.
[0048] 2. Main solution preparation method
[0049] The extraction buffer contains sodium chloride and sodium citrate, wherein the concentration of sodium chloride is 0.09-0.15M, the concentration of sodium citrate is 0.015-0.025M, the pH is 5.5-11, and the rest is water;
[0050] The balance solution contains sodium chloride and sodium citrate, wherein the concentration of sodium chloride is 0.09-0.15M, the concentration of sodium citrate is 0.015-0.025M, the pH is 6.5-7.5, and the rest is water;
[0051] The washing liquid contains sodium chloride and sodium citrate, wherein the concentration of sodium chloride is 0.3-0.5M, the concentration of sodium cit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com