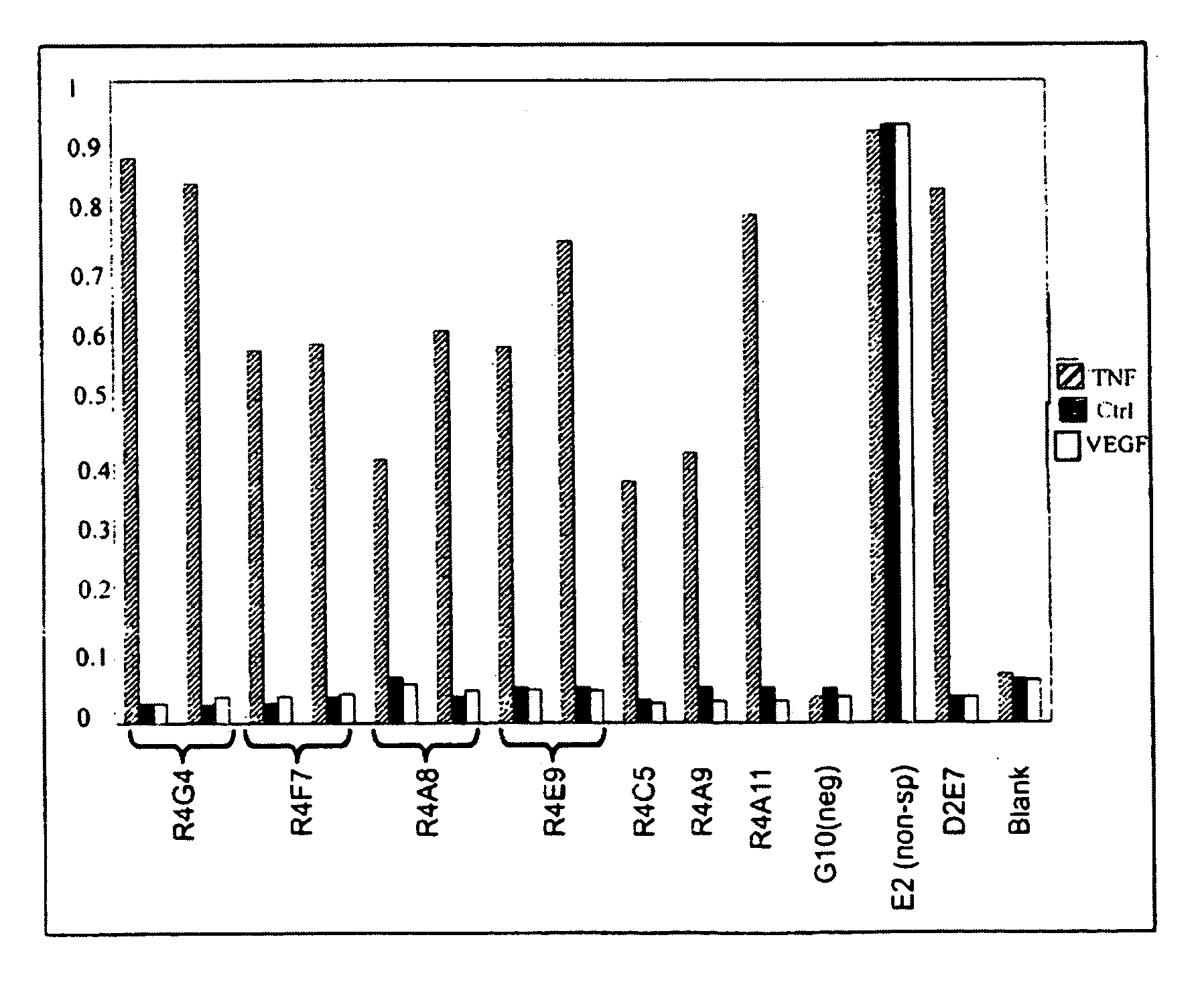
Universal fibronectin type III binding-domain libraries
a technology of binding domains and fibronectins, applied in the field of universal fibronectin type iii binding domain libraries, can solve the problems of inefficient candidate screening, excessive construction, and inability to enumerate the number of ways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods for Bioinformatic-Guided Identification of Universal Fibronectin Binding Domain Library Sequences
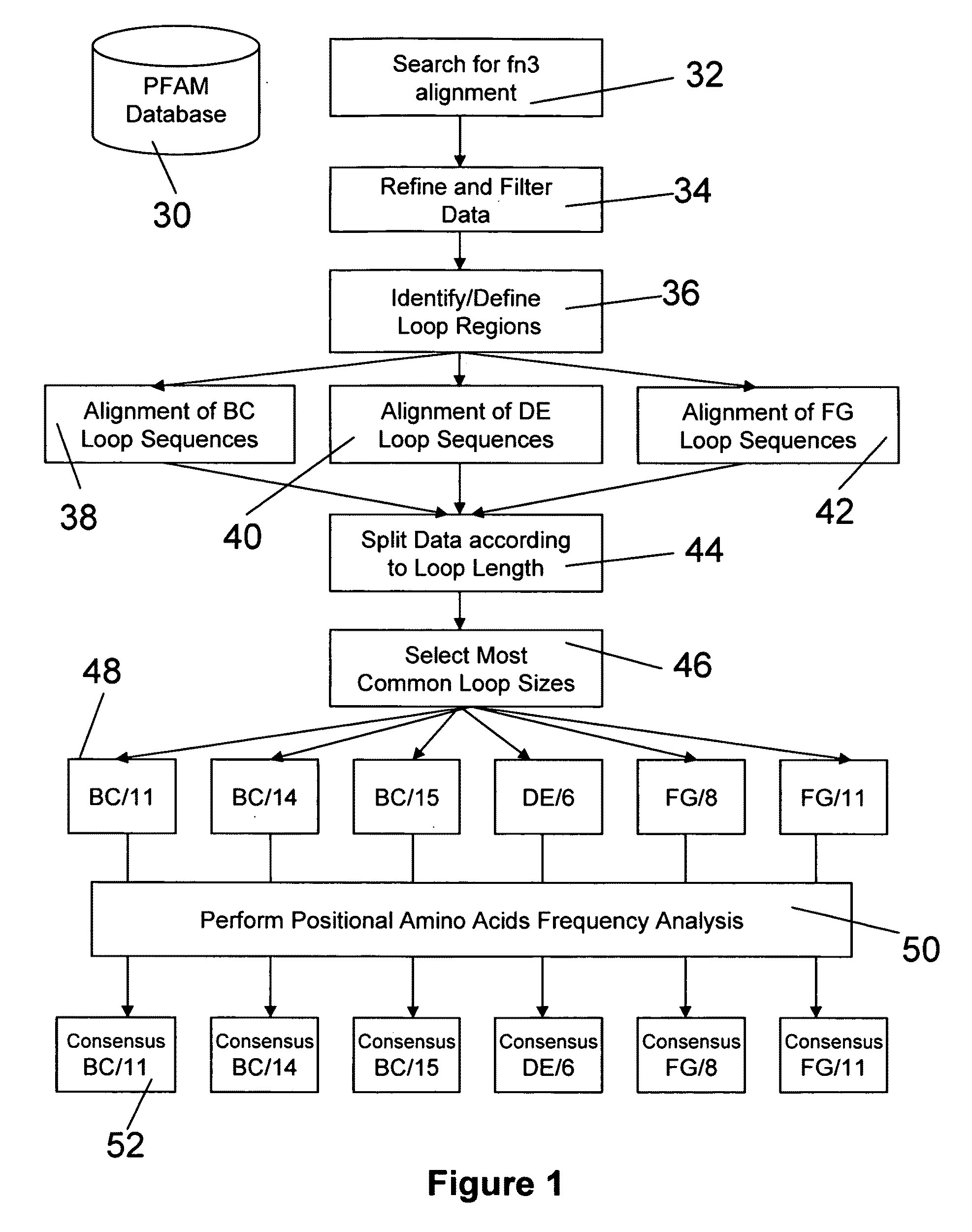
[0171]In this example, universal BC, DE, and FG loop sequences for fibronectin binding domain library sequences are identified and selected using bioinformatics and the criteria of the invention. A generalized schematic of this process is presented in FIG. 1.
[0172]Briefly, the PFAM database was searched for a multiple sequence alignment containing only sequences belonging to the Fibronectin Type III family (FN3, PFAM ID: PF00041).
[0173]This search returned an initial dataset of 9321 protein sequences. It is noted, however, that this set of sequences can increase in number as additional sequences are cloned and entered into the database. The base dataset of ˜9321 FN3 superfamily sequences included FN3 sequences from multiple sources: human fibronectin, tenascin, drosophila sevenless, protein tyrosine phosphatase, EPH tyrosine kinases, and cytokine receptors and Z proteins (see Tab...
example 2
Assessing Loop Variability Profiles Using Bioinformatics Through Filtering and Cluster Analysis of Gene Sequences
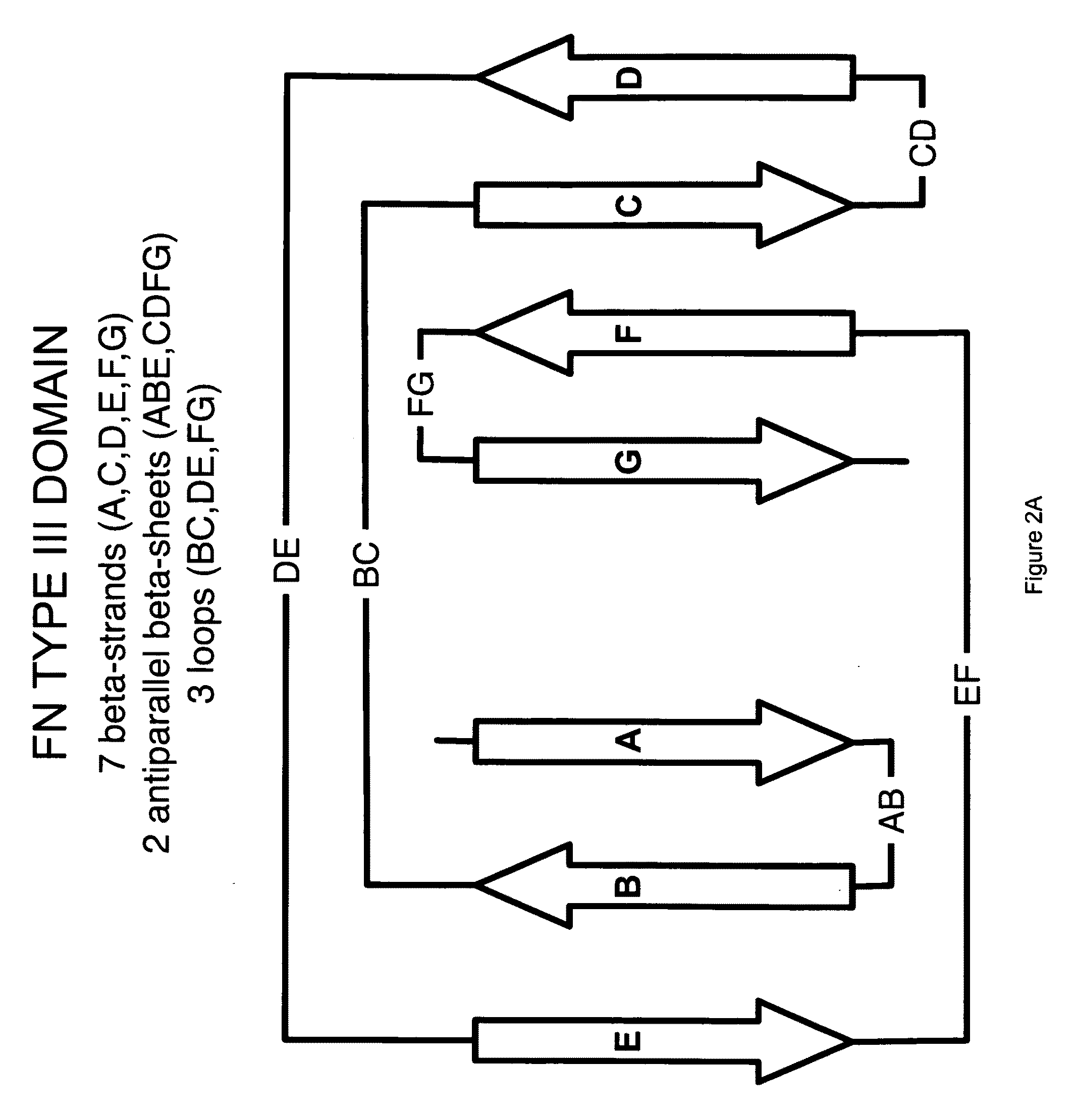
[0178]The universal fibronectin binding domain libraries were designed by determining the variability profiles for the loops expressed in vivo. The variability profiles represent the cataloging of the different amino acids, and their respective rates of occurrence, present at a particular position in a dataset of aligned sequences. Size related families of loop sequences using the parameters set forth above within this starting “base dataset” can be identified and delineated. Comparative analysis of these multiple aligned loops provide variability profile information as to the existing and “tolerated” diversity for introducing amino acid changes that can lead to potential ligand binding. The designation of loops and their comprising amino acids can also be described for other scaffold like proteins using similar definitions.
[0179]The frequency distribution of the six loop...
example 2a
Identifying Fixed and Non-Fixed Loop Positions with Thresholds
[0182]In one embodiment, a natural-variant combinatorial library with a conserved or selected semi-conserved consensus amino acid is designed as follows.
[0183]FN3 loop datasets are enumerated as above for amino acid variability and their relative frequencies at each aligned position (FIGS. 9-14). The above analysis identified positional preferences in all FN3 module loops and are termed “variability profiles.” For example, in BC loop size 11 (FIG. 9), a tryptophan (W) at position 22 is found at nearly 95% of all FN3 loop positions demonstrating high degree of selective pressure for its presence. Position 22 would be considered “conserved” for tryptophan (W22) (see below Example 3) as it occurred above a predetermined 40% threshold level and was more than twice as common as the next most frequent amino acid at that position. This “fixed” residue is seen as the dominant amino acid with respect to the other amino acids that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| threshold | aaaaa | aaaaa |
| threshold | aaaaa | aaaaa |
| frequency threshold | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com