Recombinant human fibronectin III1-C, and preparation method and application thereof
A technology of human fibronectin and III1-C, applied in the biological field, can solve the problems of long time, loss of function, complicated technology, etc., and achieve the effect of low production cost, short production cycle and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
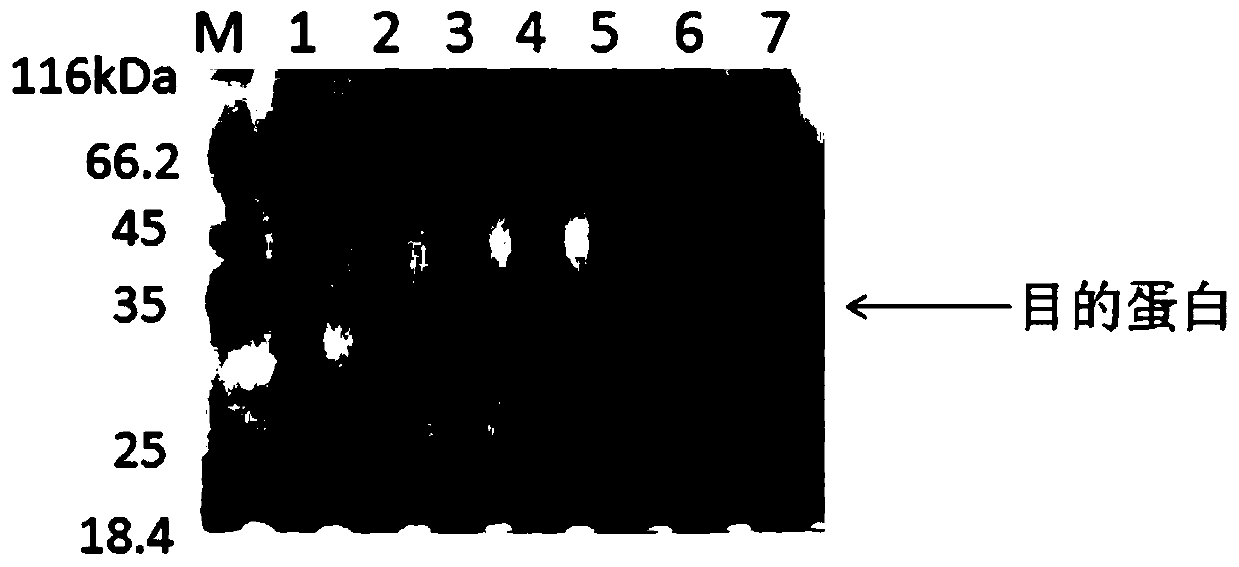
Embodiment 1
[0036] The preparation method of rhFNⅢ1-C comprises the following steps:
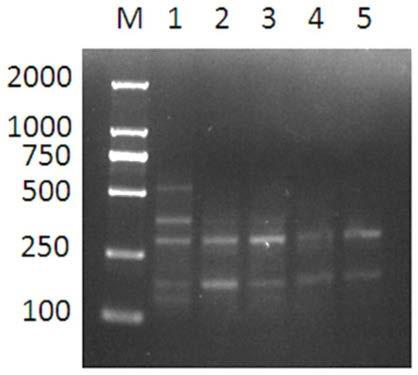
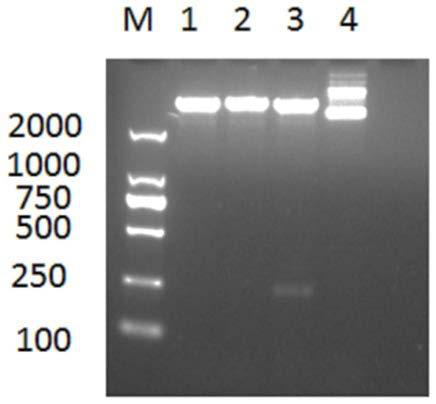
[0037] (1) With the amino acid sequence shown in SEQUENCE LISTING 400, the human fibronectin FNⅢ1-C gene fragment of the gene sequence shown in SEQUENCE LISTING 400, by adjusting the GC ratio in the gene sequence, adjusting The preferred codons of human fibronectin in Escherichia coli expression system optimize and synthesize the human fibronectin FNⅢ1-C gene fragment, and the optimized human fibronectin FNⅢ1-C gene sequence is shown as SEQUENCE LISTING 400 As shown, the upstream and downstream primer sequences of the optimized rhFNⅢ1-C gene are: upstream primer: 5'-GGATCCAACGCCCCTCAGCCG-3', downstream primer: 5'-CGGCTGAGGGGCGTTGGATCC-3';
[0038] (2) Insert the optimized rhFNⅢ1-C gene into pET-32a to construct the recombinant plasmid rhFNⅢ1-C / pET32-a;
[0039] (3) Transform the recombinant plasmid into the expression vector BL21 (DE3), and screen the positive clone bacteria on the LB plate containing ...
Embodiment 2
[0057] RhFNⅢ1-C promotes cell adhesion test, the specific steps are as follows:
[0058] (a) The purified rhFNⅢ1-C obtained in Example 1 and the positive control fibronectin (purchased from Shanghai Fibrolink Biotechnology Co., Ltd.) were diluted 10 times and 100 times with PBS buffer respectively, and added to the 96-well cell plate Dilute according to 2-fold gradient; in order to avoid the edge effect, the surrounding edge wells were not used for experiments, and a negative control was set up (only 100 μL PBS buffer was added to the negative control); incubate overnight at 4°C;
[0059] (b) 100 μL of PBS was added to each well to wash for a total of 3 times; after washing, 1% BSA was added to each well to block, that is, it was incubated in a 37° C. incubator for 1 h. After the incubation is complete, discard the liquid in the plate;
[0060] (c) Preparation of MDBK single cell suspension in good growth state. Adjust the density of MDBK single cell suspension to 1.0×10 / mL,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com