Rapidly-dissolving and stabile montelukast oral solid preparation and preparation method thereof
A technology of montelukast sodium and solid preparation, which is applied in the field of leukotriene receptor antagonists, can solve the problems of increase in substance, decrease in content, inequivalence, etc., so as to improve the dissolution rate, improve the stability, and avoid unequal effect of risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
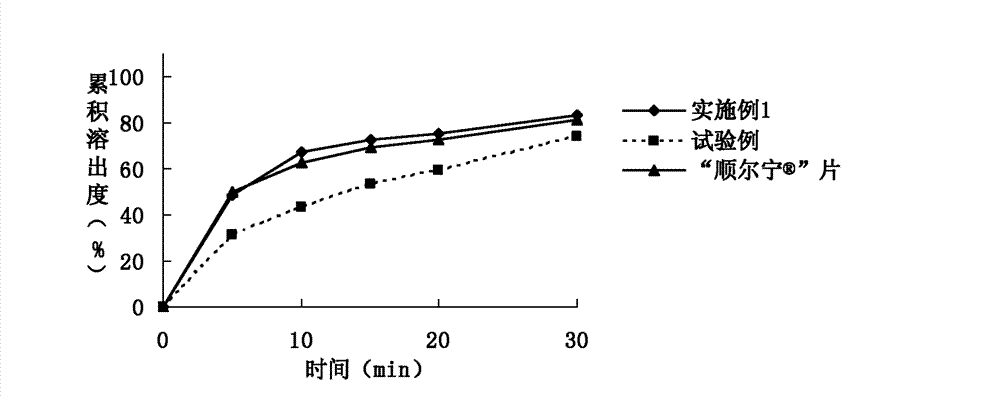
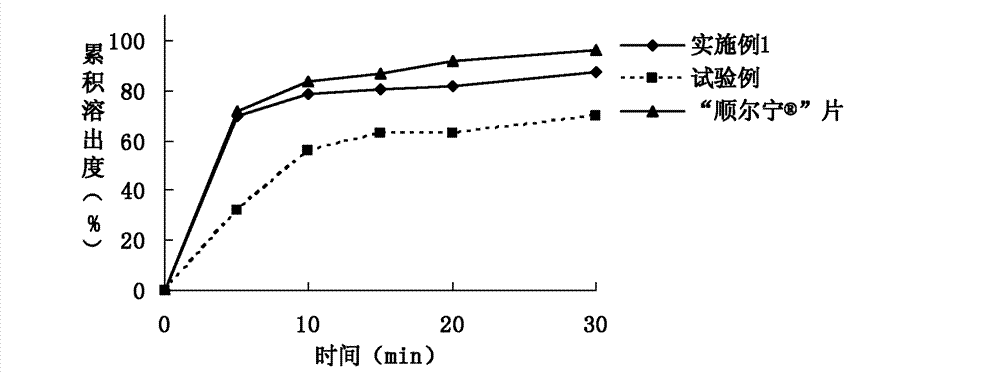
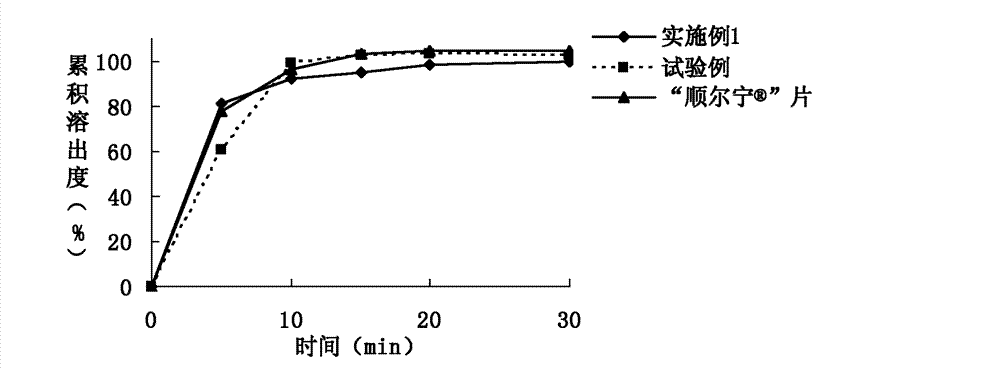
[0076] Example 1. Prescription composition of montelukast sodium tablets: specification 10 mg (calculated as montelukast), prescription quantity is 1000 tablets.
[0077]
[0078] Preparation:
[0079] Take the prescribed amount of montelukast sodium and pass through a 100-mesh sieve, and microcrystalline cellulose, lactose, croscarmellose sodium, hyprolose, and magnesium stearate pass through a 60-mesh sieve respectively;
[0080] Get the sodium hydroxide of prescription quantity, add appropriate amount of water to be mixed with 0.5% sodium hydroxide aqueous solution, set aside.
[0081] Put the above-mentioned montelukast sodium, microcrystalline cellulose, lactose, croscarmellose sodium, and hyprolose into a wet mixing granulator and mix evenly; Add the prepared amount of 0.5% sodium hydroxide aqueous solution into the machine, high-speed shear granulation, oven-dry at 50-60°C, and granulate; then add magnesium stearate, and mix in a mixer. Tablet.
[0082] Opadry coa...
Embodiment 2
[0083] Example 2. Prescription composition of montelukast sodium tablets: specification 10 mg (calculated as montelukast), prescription quantity is 1000 tablets.
[0084]
[0085] Preparation:
[0086] Take the prescribed amount of montelukast sodium and pass through a 100-mesh sieve, and microcrystalline cellulose, lactose, croscarmellose sodium, hyprolose, sodium carbonate, and magnesium stearate pass through a 60-mesh sieve respectively;
[0087] Put the above-mentioned montelukast sodium, microcrystalline cellulose, lactose, croscarmellose sodium, hydroxypropyl cellulose, and sodium carbonate in a mixer and mix evenly, then add magnesium stearate and mix . Direct mixing powder compression tablet.
[0088] Opadry coating powder 32K12968 (coating material) is used to coat the obtained montelukast sodium tablets, and the consumption of Opadry coating powder 32K12968 is 4% of the total amount of the above-mentioned montelukast sodium tablets, namely 8.00 g, coated to mak...
Embodiment 3
[0089] Embodiment 3. Montelukast Sodium Chewable Tablets Prescription Composition: Specification 5mg (calculated as Montelukast), prescription quantity is 1000 tablets
[0090]
[0091] Preparation:
[0092] Take the prescribed amount of montelukast sodium and pass through a 100-mesh sieve, red iron oxide through a 80-mesh sieve, mannitol, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, cherry flavor, aspartame and magnesium stearate respectively through a 60 mesh sieve;
[0093] Get the sodium hydroxide of prescription quantity, add appropriate amount of water to be mixed with 0.7% sodium hydroxide aqueous solution, set aside.
[0094] Put the above-mentioned montelukast sodium, mannitol, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, red iron oxide, cherry essence and aspartame in a wet mixing Mix evenly in the granulator; add the prepared amount of 0.7% sodium hydroxide aqueous solution into the wet mixing granul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com