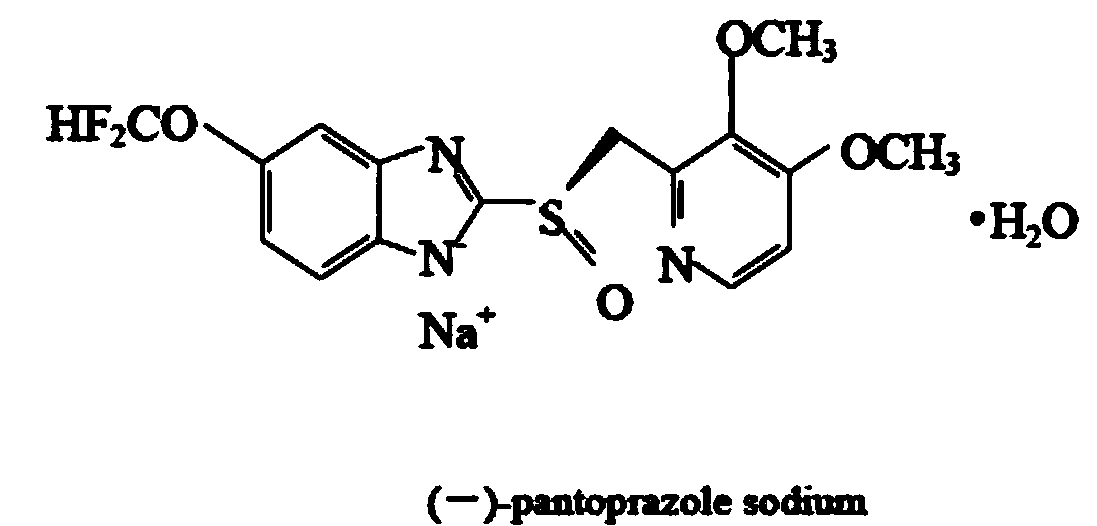
(-)-pantoprazole sodium enteric coated tablet and preparation method thereof
A technology of levopantoprazole sodium and enteric-coated tablets, applied in the field of medicine, can solve the problems of low bioavailability, poor compatibility of excipients, poor disintegration, etc., and achieve high bioavailability, fast dissolution rate, good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
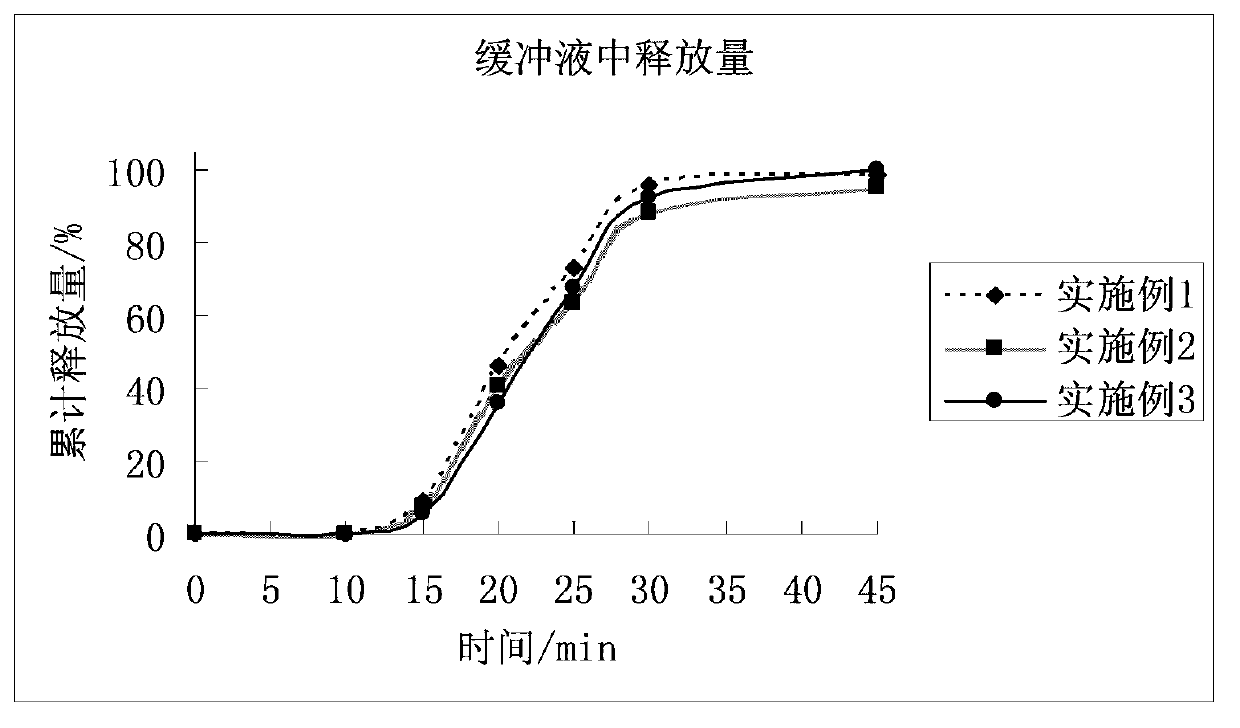
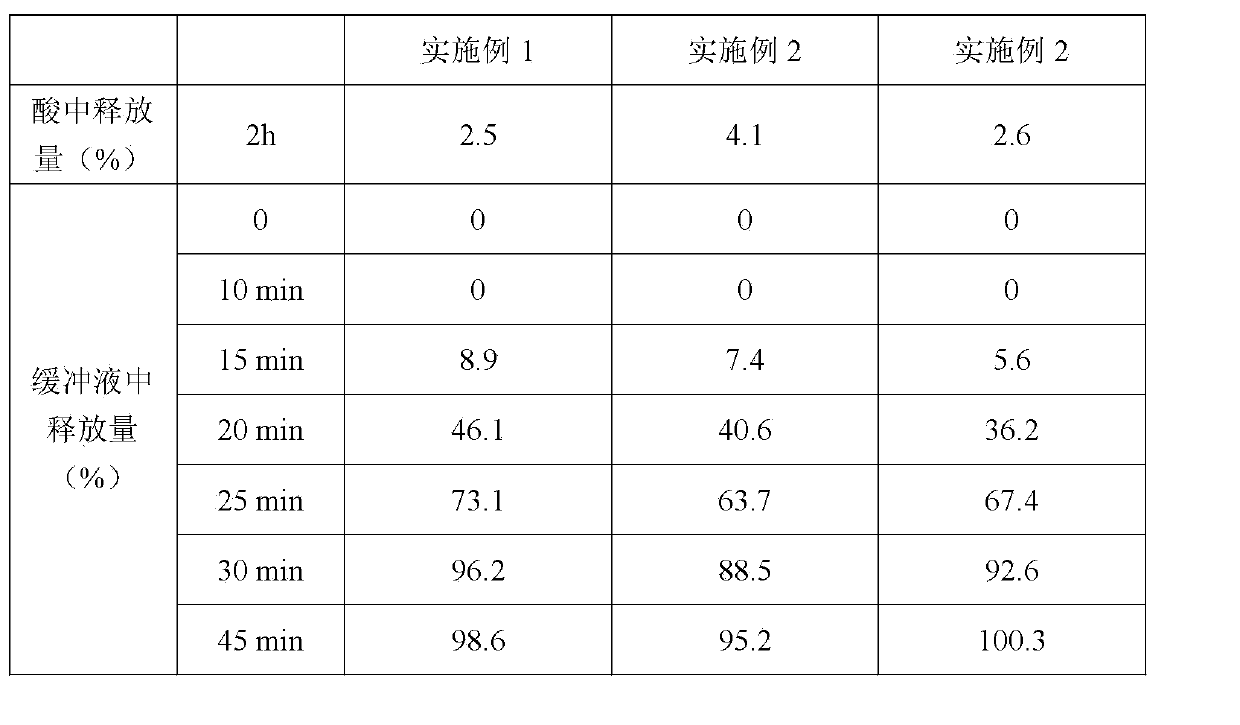
Embodiment 1
[0023] (1) chip
[0024] Tablet prescription:
[0025] Material name Dosage L-pantoprazole sodium 1.5 hydrate 22.6g Anhydrous Sodium Carbonate 15.0g Mannitol 94.0g Croscarmellose Sodium 5.6g PVP K30 1.4g Magnesium stearate 1.4g 1000 pieces
[0026] [0026] Preparation of tablet cores:
[0027] The croscarmellose sodium of levopantoprazole sodium 1.5 hydrate and prescription quantity 80% anhydrous sodium carbonate, mannitol and prescription quantity 60% is carried out dry mixing, puts into the wet granulator after mixing In a container, set aside. Dissolve PVP K30 and the remaining anhydrous sodium carbonate in water to make a granulation solution, and add the granulation solution to the mixture in the container of the wet granulator under stirring and shearing to prepare granules. Dry the granules and mix them with the remaining croscarmellose sodium and magnesium stearate, and then compress the mixture into ta...
Embodiment 2
[0039] (1) chip
[0040] Tablet prescription:
[0041] Material name Dosage L-pantoprazole sodium 1.5 hydrate 22.6g sodium bicarbonate 25.0g lactose 82.8g Sodium carboxymethyl starch 7.0g HPMC 2910 1.6g Magnesium stearate 1.0g 1000 pieces
[0042] [0042] Preparation of tablet cores:
[0043] Dry mix levopantoprazole sodium 1.5 hydrate with 70% sodium bicarbonate, lactose and sodium carboxymethyl starch, mix well and put it into the container of the wet granulator for later use, mix HPMC 2910 with the remaining carbonic acid Sodium hydrogen is dissolved in water to form a granulation liquid, and the granulation liquid is added to the above mixture under stirring and shearing to prepare granules. Dry the granules and mix them with magnesium stearate. After mixing evenly, the mixture is compressed to obtain tablet cores. The weight of each prepared tablet core is about 140 mg, corresponding to 20 mg of L-pantopraz...
Embodiment 3
[0055] (1) chip
[0056] Tablet prescription:
[0057] Material name Dosage L-pantoprazole sodium 1.5 hydrate 11.3g Anhydrous Sodium Carbonate 10.0g Mannitol 30.0g lactose 42.6g PVPP 4.5g PVP K90 0.6g Magnesium stearate 1.0g 1000 pieces
[0058] [0058] Preparation of tablet cores:
[0059] Levopantoprazole sodium 1.5 hydrate and the PVPP of 60% anhydrous sodium carbonate, mannitol, lactose and prescription quantity 80% of prescription quantity are carried out dry mixing, put into the container of wet granulator after mixing, will PVP K90 and the remaining anhydrous sodium carbonate are dissolved in water to form a granulation liquid, and the granulation liquid is added to the above mixture under stirring and shearing to prepare granules. Dry the granules and mix them with the remaining PVPP and magnesium stearate. After mixing evenly, the mixture is compressed into tablets to obtain tablet cores. The weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com