Amorphous Venetoclax and pharmaceutic adjuvant solid dispersoid and preparation method thereof
A technology of solid dispersion and pharmaceutical excipients, which is applied in the direction of pharmaceutical formulations, medical preparations containing non-active ingredients, and medical preparations containing active ingredients, etc., and can solve problems affecting drug bioavailability and reducing the solubility of active ingredients, etc. To achieve the effect of improving bioavailability, increasing stability and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
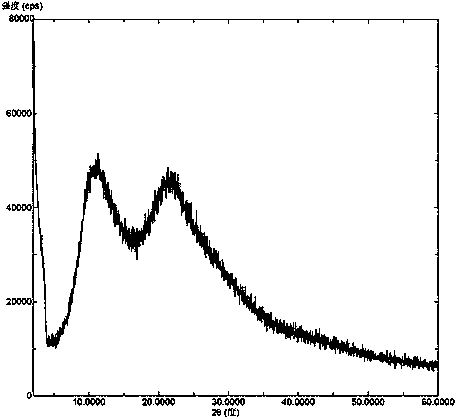
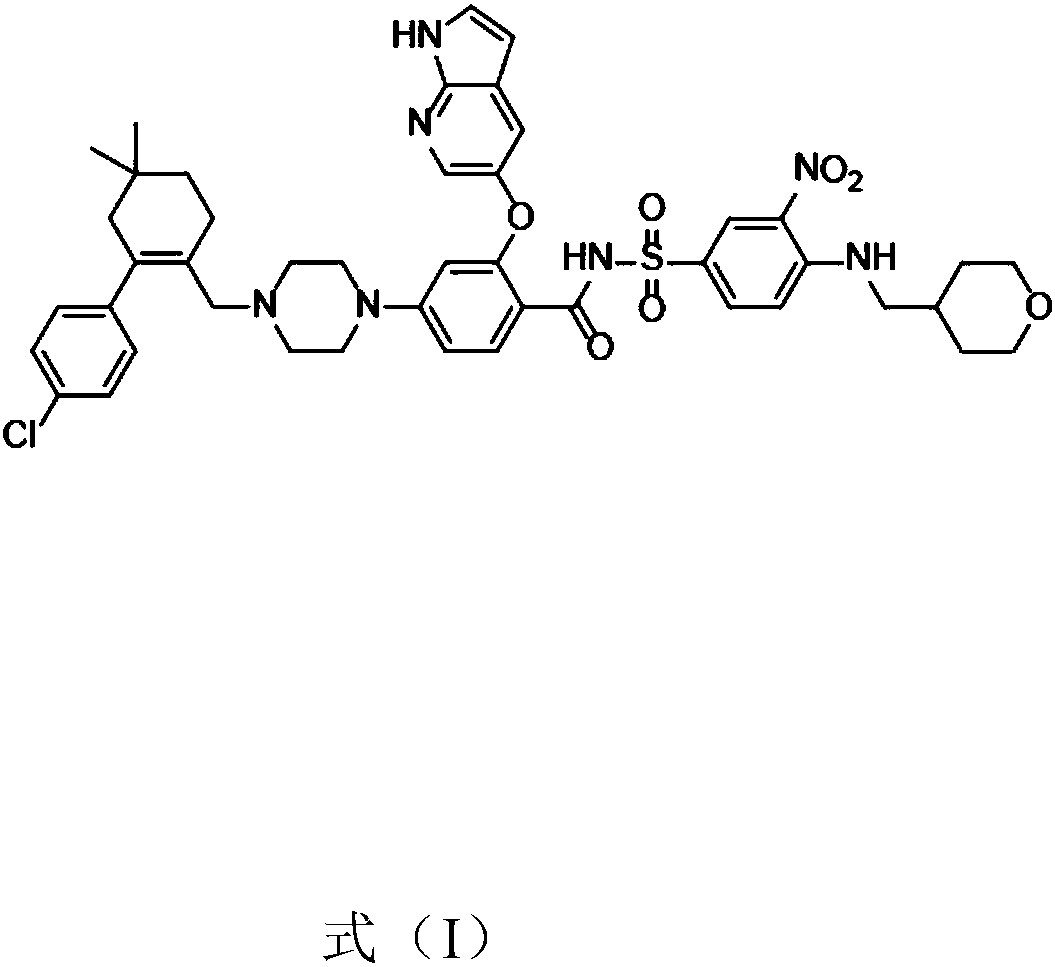
[0041] Add Venetoclax (5 g) and povidone K30 (10 g) into water (300 ml), heat to 60° C. and stir to dissolve. Dry the above solution with a JISL micro-spray dryer LSD-48, maintain the inlet temperature at 60°C and the outlet temperature at 50°C, collect the outlet material to obtain a white solid, and further vacuum-dry to obtain a solid dispersion of amorphous Venetoclax and povidone-K30 . X-ray powder diffraction pattern as figure 1 As shown, in the X-ray powder diffraction pattern of the solid dispersion, there is no characteristic peak of the Venetoclax crystal form after deducting the background peak of the pharmaceutical excipient.
Embodiment 2
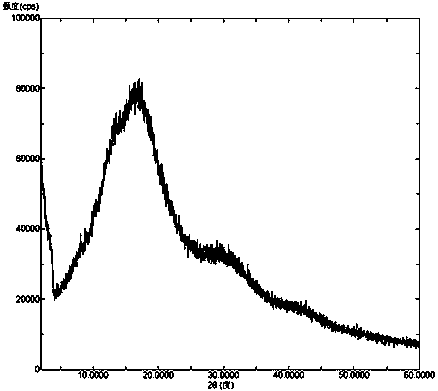
[0043] Add Venetoclax (1 g) and hydroxypropylmethylcellulose E50 (0.2 g) into water (10 ml), heat to 40°C and stir to dissolve. The above solution was freeze-dried to obtain a white solid, that is, a solid dispersion of amorphous Venetoclax and hydroxypropylmethylcellulose E50. In the X-ray powder diffraction pattern of the solid dispersion, there was no Venetoclax after deducting the background peaks of pharmaceutical excipients The characteristic peaks of the crystal form.
Embodiment 3
[0045] Venetoclax (1 g) and polyethylene glycol 8000 (50 g) were heated to melt, and rapidly cooled to room temperature with stirring to obtain a white solid. The above solid was pulverized to obtain a white powdery solid, i.e. a solid dispersion of amorphous Venetoclax and polyethylene glycol 8000. In the X-ray powder diffraction pattern of the solid dispersion, there was no Venetoclax crystal after deducting the background peak of pharmaceutical excipients. type of characteristic peaks.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com