Albendazol dispersible tablets and preparation method thereof
A kind of technology of albendazole and dispersible tablets, applied in the field of albendazole dispersible tablets and preparation thereof, and can solve the problems such as not finding albendazole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
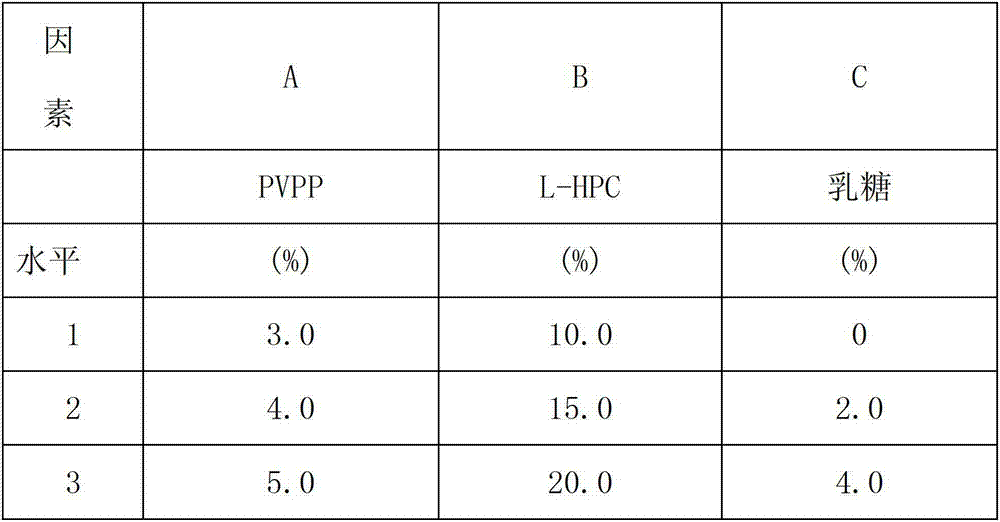
[0074] Example 1: The dispersible tablets include the following raw materials in weight ratio per 1000 tablets: 200g of albendazole, 16g of polyvinylpolypyrrolidone, 80g of low-substituted hydroxypropyl cellulose, 60g of microcrystalline cellulose, and 8g of lactose , sodium lauryl sulfate 8g, stevia 12g, micronized silica gel 12g, magnesium stearate 4g;
[0075] Above-mentioned albendazole dispersible tablet, its preparation process is:
[0076] a. Material preparation: Weigh the prescribed amount of albendazole, microcrystalline cellulose, crospovidone, low-substituted hydroxypropyl cellulose, lactose and other materials, grind them, pass through a 100-mesh sieve, and put them in a clean container , sealed and preserved.
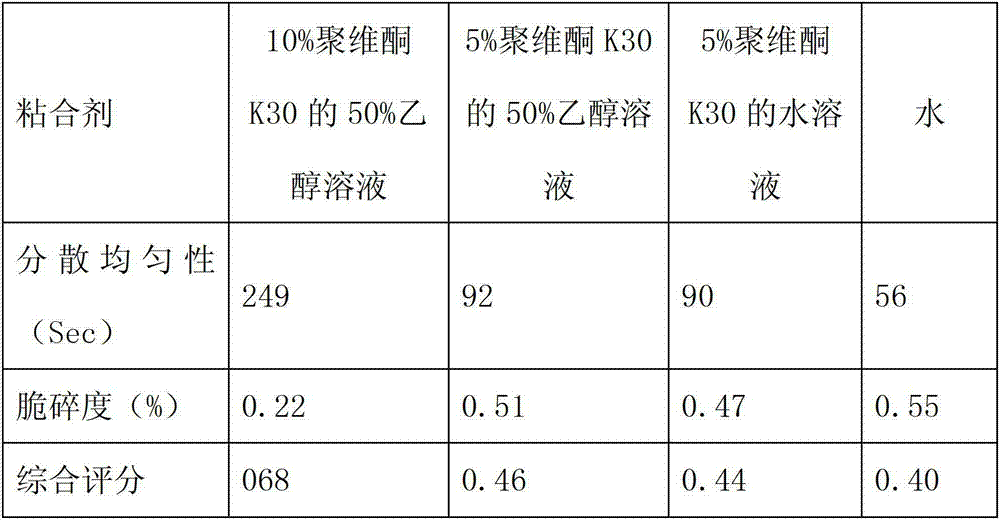
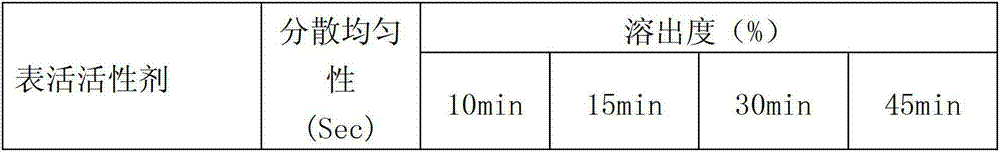
[0077] b. Take the sodium lauryl sulfate and stevioside of the prescribed amount, dissolve them in 14 times the amount of water, and use them as wetting agents for later use.
[0078] C, granulation: take albendazole fine powder, mix with the crospovidon...
Embodiment 2
[0082] Embodiment 2: every 1000 tablets of this dispersible tablet include the raw materials of following weight ratio: albendazole 200g, crospovidone 80g, croscarmellose sodium 20g, microcrystalline cellulose 60g, lactose 8g, sodium lauryl sulfate 8g, stevia 12g, micronized silica gel 8g, magnesium stearate 4g;
[0083] The preparation technology of above-mentioned albendazole dispersible tablet is identical with embodiment 1.
Embodiment 3
[0084] Embodiment 3: every 1000 tablets of this dispersible tablet include the raw materials of the following weight ratio: 200g of albendazole, 60g of crospovidone, 24g of low-substituted hydroxypropyl cellulose, croscarmellose sodium 20g, microcrystalline cellulose 50g, lactose 10g, sodium lauryl sulfate 12g, stevia 16g, micropowder silica gel 4g, magnesium stearate 4g;
[0085] The preparation technology of above-mentioned albendazole dispersible tablet is identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com