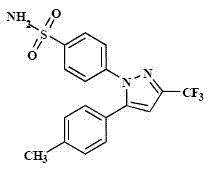
Celecoxib solid composition with high dissolution, preparation method and application
A solid composition, a technology of celecoxib, which is applied in the directions of non-active components of polymer compounds, pharmaceutical combinations, medical preparations of non-active components, etc. The problems such as low dissolution rate of lecoxib can achieve the effect of good preparation performance, simple preparation method and fast dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of celecoxib solid composition with high dissolution rate
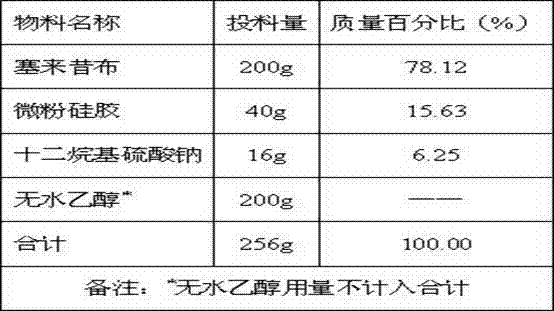
[0036] Table 1: Preparation of solid composition A1
[0037]
[0038] Add absolute ethanol into a container containing celecoxib, heat to 70~80°C to obtain a clear solution, absorb the solution with micropowder silica gel, dry at 60~70°C, and remove ethanol to obtain a solid composition of celecoxib A1.
[0039] Table 2: Preparation of solid composition A2
[0040]
[0041] Add sodium lauryl sulfate to absolute ethanol, heat to 70~80°C to obtain a clear solution, add celecoxib, dissolve to obtain a clear solution, absorb with micropowder silica gel, dry and evaporate at 60~70°C Celecoxib solid composition A2 obtained from ethanol.
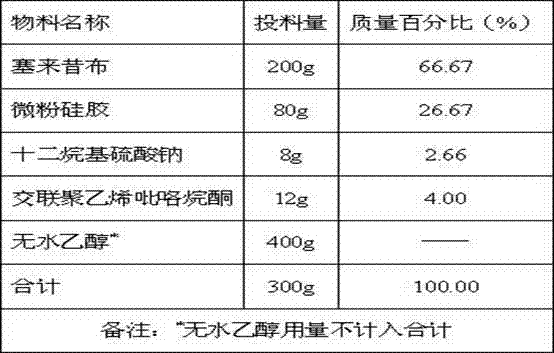
[0042] Table 3: Preparation of solid composition A3
[0043]
[0044] Add sodium lauryl sulfate to absolute ethanol, heat to 70~80°C to obtain a clear solution, add celecoxib, dissolve to obtain a clear solution, absorb with micropowder silica g...
Embodiment 2
[0061] Embodiment 2: Preparation of oral solid preparation
[0062] Preparation of capsules
[0063] Take 28.00 g of the solid composition A1, pulverize it, pass through an 80-mesh sieve, and pack it into 100 capsules to obtain capsules containing 200 mg of celecoxib.
[0064] Take 28.80 grams of the solid composition A2, pulverize it, pass through an 80-mesh sieve, and pack it into 100 capsules to obtain capsules containing 200 mg of celecoxib.
[0065] Take 25.60 grams of the solid composition A3, pulverize it, pass through an 80-mesh sieve, and pack it into 100 capsules to obtain capsules containing 200 mg of celecoxib.
[0066] Take 30.02 grams of the solid composition A4, pulverize it, pass through an 80-mesh sieve, and pack it into 100 capsules to obtain capsules containing 200 mg of celecoxib.
[0067] Take 29.20 grams of the solid composition A5, pulverize it, pass through an 80-mesh sieve, and pack it into 100 capsules to obtain capsules containing 200 mg of cel...
Embodiment 3
[0076] Embodiment 3: the preparation of tablet
[0077] Table 10: A1 tablet
[0078] Material name Sample weight (g) mass percentage A1 28.00 87.50% Crospovidone 1.25 3.91% Polyvinylpyrrolidone 1.00 3.13% Sodium dodecyl sulfate 1.25 3.91% Micropowder silica gel 0.15 0.47% Magnesium stearate 0.35 1.09% water Appropriate amount —— total 32.00 100.00%
[0079] Weigh raw and auxiliary materials according to Table 10, dissolve sodium lauryl sulfate and polyvinylpyrrolidone in water and configure an aqueous solution as an adhesive for standby use, take material A1 and use adhesive to make soft material, granulate, and pass through a 40-mesh sieve , dried, granulated, added cross-linked povidone, micro-powder silica gel, magnesium stearate, mixed evenly, and pressed into 320mg / tablet.
[0080] Table 11: A2 Tablets
[0081] Material name Sample weight (g) mass percentage A2 28.80 90.00% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com