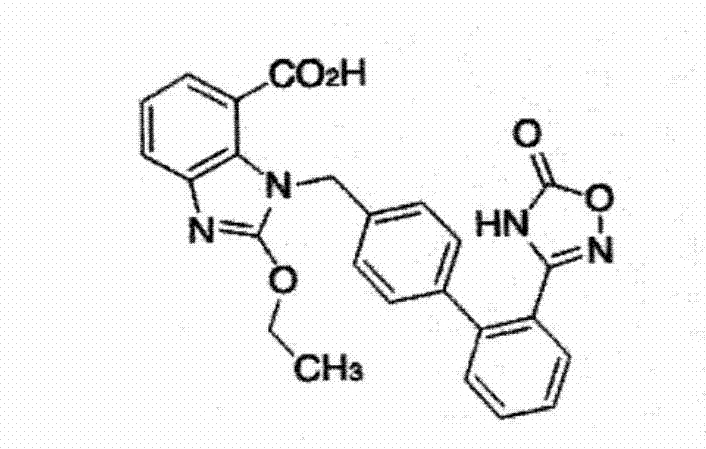
Azilsartan tablet and preparation method thereof
An azilsartan tablet and tablet technology, applied in the field of medicine, achieves the effects of good stability, simple process, and fast dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
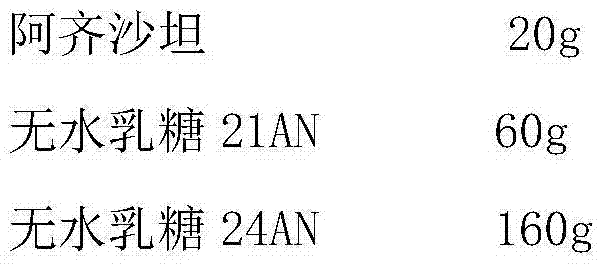
[0027]
[0028] Preparation Process:
[0029] (1) Azilsartan is micronized, D90 is controlled to be less than 20 microns, and the prescribed amount is weighed;
[0030] (2) Anhydrous lactose 21AN was passed through a 65-mesh sieve, and the particles were removed by sieving, and the recipe quantity was weighed;
[0031] (3) Mix (1) and (2) evenly, add lactose anhydrous 24AN, magnesium stearate, and crospovidone in the recipe quantity, mix well, and press into tablets.
Embodiment 2
[0033]
[0034]
[0035] Preparation Process:
[0036] (1) Azilsartan is micronized, D90 is controlled to be less than 20 microns, and the prescribed amount is weighed;
[0037] (2) Anhydrous lactose 21AN was passed through a 65-mesh sieve, and the particles were removed by sieving, and the recipe quantity was weighed;
[0038] (3) Mix (1) and (2) evenly, add anhydrous lactose 24AN, magnesium stearate, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose, silicon dioxide of the recipe quantity and mix well, press into tablets .
Embodiment 3
[0040]
[0041] Preparation Process:
[0042] (1) Azilsartan is micronized, D90 is controlled to be less than 20 microns, and the prescribed amount is weighed;
[0043] (2) Anhydrous lactose 21AN was passed through a 65-mesh sieve, and the particles were removed by sieving, and the recipe quantity was weighed;
[0044] (3) Mix (1) and (2) evenly, add lactose anhydrous 24AN, magnesium stearate, and crospovidone in the recipe quantity, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com