Crystal form, preparing method and application of Olaparib
A technology of crystal forms and mixed crystals, which is applied in organic chemical methods, pill delivery, organic chemistry, etc., can solve the problems of unfavorable olaparib dissolution and large particle size, and achieve improved drug efficacy, simple preparation method, and stable performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The present invention also provides a method for preparing the crystalline form of olaparib provided by the present invention, comprising:
[0038] 1) olaparib is mixed with a solvent and refluxed to obtain a olaparib solution,
[0039] The solvent is one or more of n-propanol, isopropanol, n-butanol, isobutanol and tert-butanol;
[0040] 2) Filtrating the olaparib solution and cooling to obtain the olaparib in the crystal form.
[0041]According to the present invention, the present invention mixes olaparib with solvent and refluxes to obtain a saturated solution of olaparib; the solvent is preferably one or more of n-propanol, isopropanol, n-butanol and tert-butanol species; the dosage ratio of the olaparib to the solvent is preferably 1g:(8-20)mL, more preferably 1g:(10-15)mL, most preferably 1g:(12-14)mL.
[0042] The present invention also filters the obtained olaparib solution, cools, and dries to obtain the olaparib of the crystal form; the filtering is prefera...
Embodiment 1
[0052] Add 20.0 g of olaparib (purity 99.1%) and 200 mL of isopropanol into the reaction flask, raise the temperature to reflux, stir for 30 min, and filter while hot. The filtrate is naturally cooled to room temperature for crystallization under stirring, filtered, and depressurized at 50 ° C. After drying, 17.6 g of olaparib with a new crystal form was obtained, with a yield of 88.0% and a purity of 99.9%.
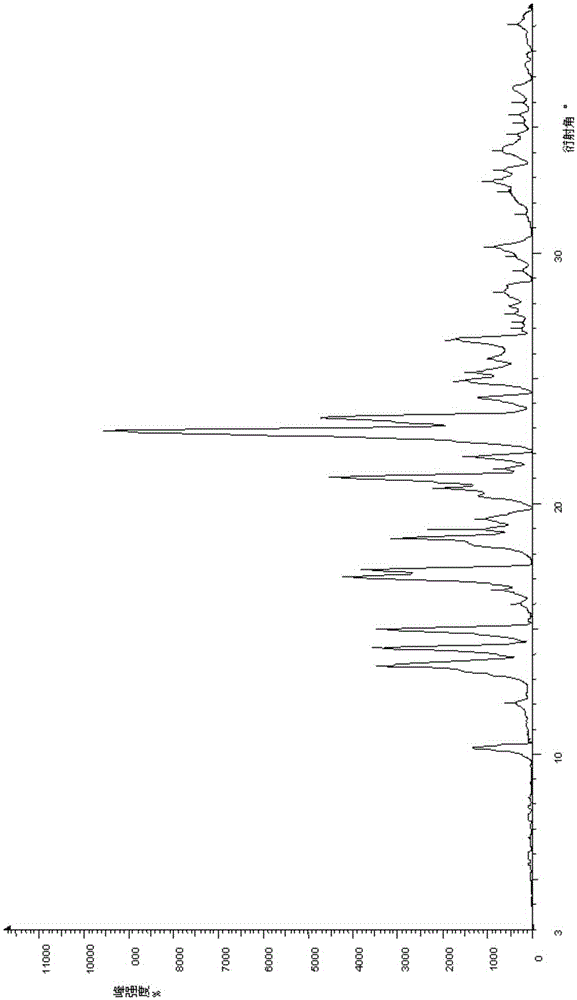
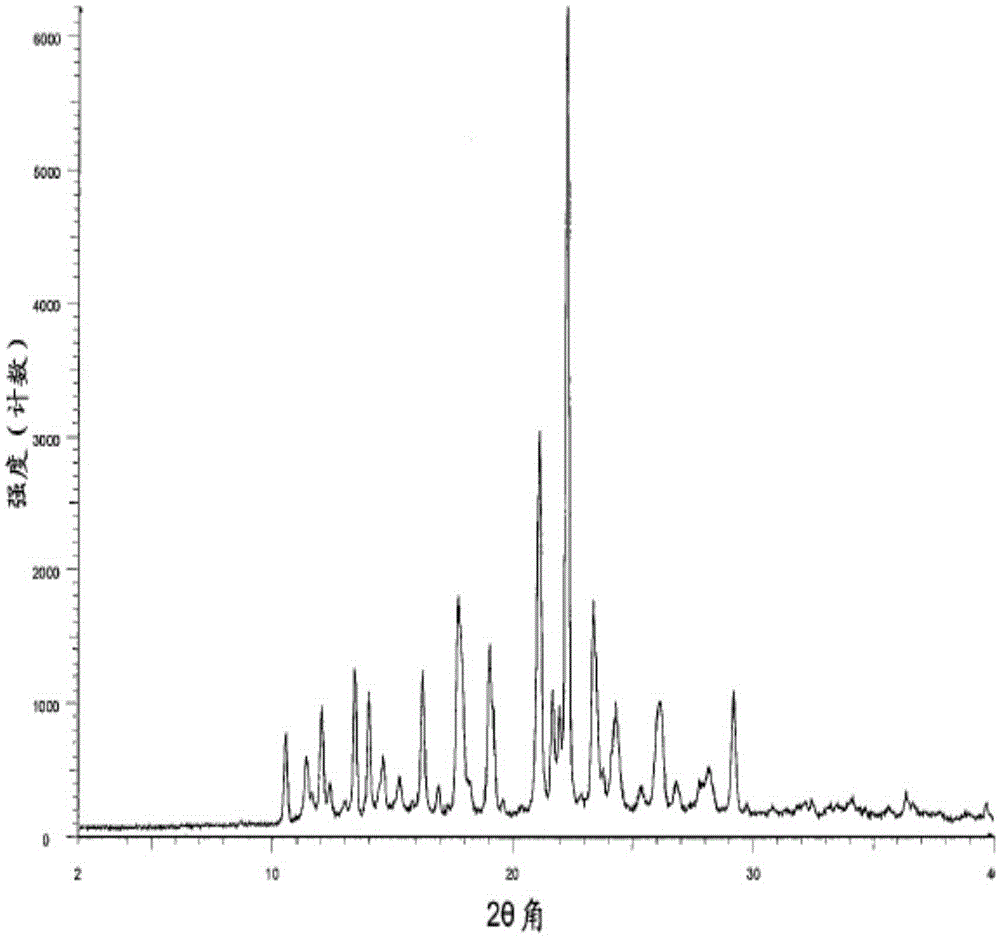
[0053] By performing X-ray powder diffraction on the new crystal form Olaparib prepared in Example 1, the results are shown in figure 1 , figure 1 The X-ray diffraction pattern of the new crystal form olaparib prepared in Example 1 of the present invention;
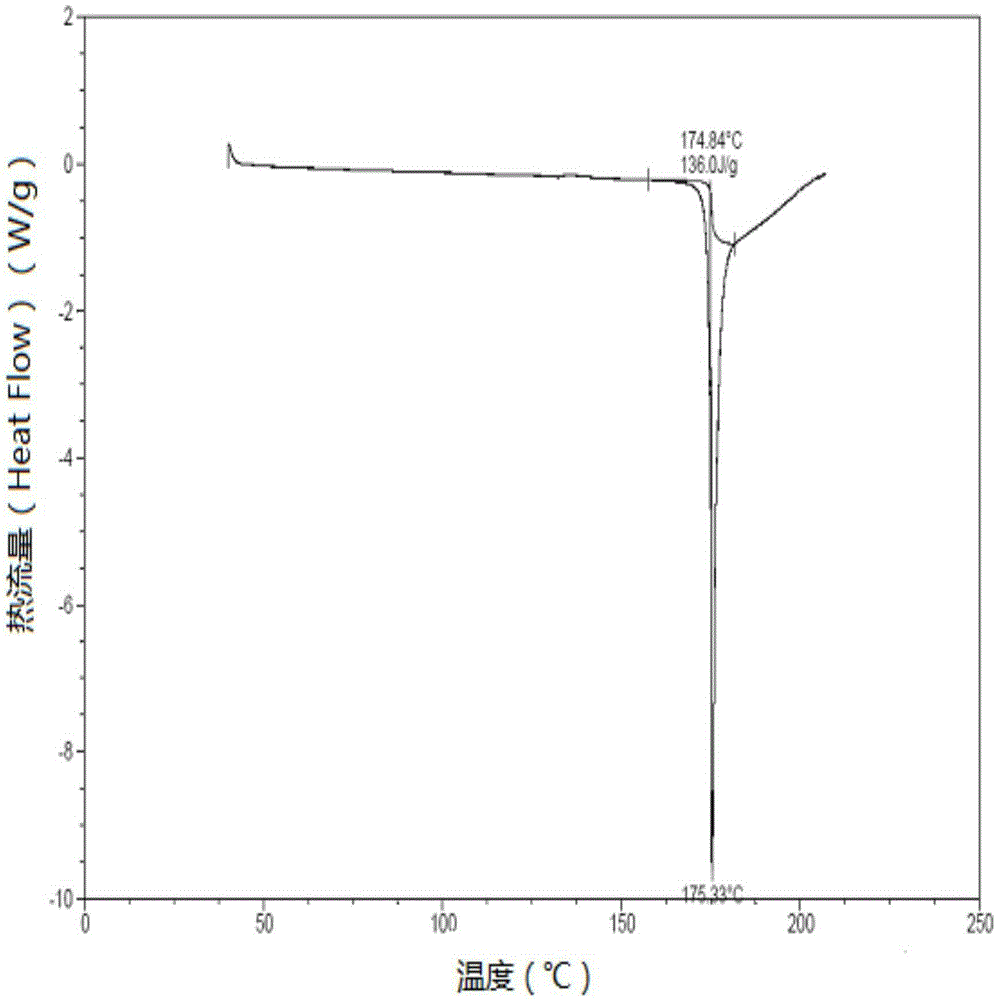
[0054] By performing differential thermal analysis on the new crystal form olaparib prepared in Example 1, the results are shown in figure 2 , figure 2 The DSC chart of the new crystal form of olaparib was prepared for Example 1 of the present invention.
[0055] The stability of the new crystal form of olapar...
Embodiment 2
[0067] Add 20.0g of olaparib and 300mL of tert-butanol into the reaction flask, raise the temperature to 100°C, stir for 30min, and filter while it is hot. The filtrate is naturally cooled to room temperature for crystallization under stirring, filtered, and dried under reduced pressure at 50°C to obtain 17.9g The new crystal form of olaparib has a yield of 89.5% and a purity of 99.9%.
[0068] X-ray powder diffraction was performed on the obtained crystal form olaparib, and the results showed that the diffraction peak position of olaparib obtained in Example 2 was the same as that in Example 1, and it was the same crystal form.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com