Detection method of Rivaroxaban tablet relevant substances
A detection method and rivaroxaban technology, applied in the field of medicine, can solve the problems of no detection method for rivaroxaban tablets, large differences in polarity, no reservation, etc., to ensure safety and effectiveness, and low cost , the effect of short analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Instruments and Conditions
[0038] time (min) Buffer salt (%) Acetonitrile (%) 0 82 18 5 82 18 12 60 40 40 60 40 42 82 18 54 82 18
[0039] Experimental steps:
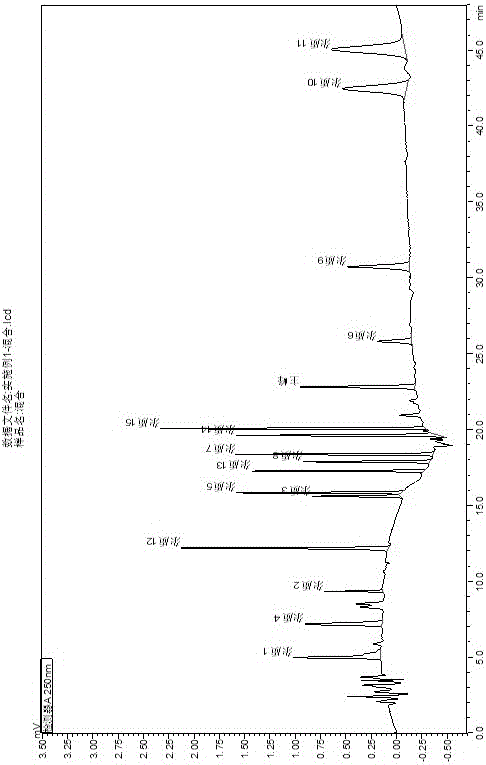
[0040] keep time Compound name Peak area Number of plates Separation Tailing factor 4.968 Impurity 1 9355 7035 -- 2.172 7.172 Impurity 4 9087 7865 7.864 1.114 9.330 Impurity 2 2341 101310 9.795 1.203 12.186 Impurity 12 13405 72689 19.167 0.821 15.596 Impurity 3 5449 120991 18.936 1.018 15.828 Impurity 5 9129 152020 1.358 1.119 17.258 Impurities 13 8469 222138 9.259 1.159 17.873 Impurity 8 7580 170485 3.851 1.111 18.354 Impurities 7 10924 209920 2.881 1.099 19.605 Impurity 14 13609 169341 7.135 1.105 20.056 Impurity 15 14938 240467 2.548 1.157 22.800 Rivaroxaban 10186 136088 13.355 1.075 25.808 Impurity 6 4144 994...
Embodiment 2
[0043] Instruments and Conditions
[0044] time (min) Buffer salt (%) Acetonitrile (%) 0 82 18 5 82 18 12 60 40 40 60 40 42 82 18 54 82 18
[0045] Experimental steps:
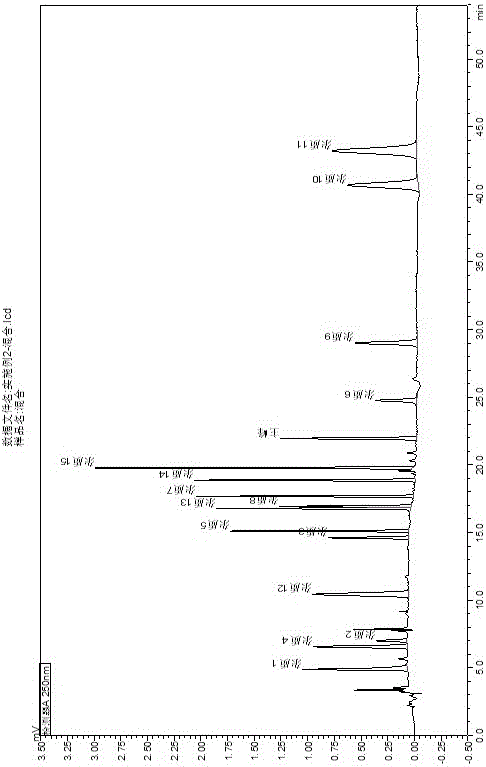
[0046] keep time Compound name Peak area Number of plates Separation Tailing factor 4.870 Impurity 1 9073 7072 -- 1.676 6.552 Impurity 4 9118 8576 6.538 1.070 6.992 Impurity 2 2444 13576 1.683 1.050 10.424 Impurity 12 13619 9789 10.376 1.076 14.602 Impurity 3 5510 82652 13.378 1.064 15.101 Impurity 5 9635 133130 2.706 1.081 16.771 Impurities 13 9855 189887 10.455 -- 16.929 Impurity 8 7634 129988 0.921 -- 17.665 Impurities 7 10992 204863 4.283 1.071 18.859 Impurity 14 13025 178741 7.139 1.055 19.771 Impurity 15 17272 227974 5.300 1.037 21.945 Rivaroxaban 9949 162685 11.345 1.054 24.758 Impurity 6 4439 111896 10.9...
preparation example 1
[0054] Preparation Example 1 Preparation of Rivaroxaban Impurity 6
[0055]
[0056]22.6g (111.21mmol) S-(+)-N-(2,3-ethoxypropyl)phthalimide (SM1), 5.34g (27.80mmol) 4-(4-aminobenzene Base) morpholin-3-one (SM2) was sequentially added into a 250ml three-neck flask, then 110ml of absolute ethanol was added, and the temperature was raised to reflux for 24h. Cool down to 50~55°C with tap water, filter with suction to remove insoluble matter, collect the filtrate, continue to cool down to 10~15°C to crystallize, filter to obtain a yellow solid. The yellow solid was dissolved with 80ml of absolute ethanol under reflux, cooled to 5-10°C, filtered, and the filter cake was dried under reduced pressure at 50-60°C for 5 hours. 10.0 g of the product was obtained with a yield of 60.0% and a purity of 96.2%. h 1 -NMR (600HZ, DMSO-d6): 7.86-7.87 (4H, m), 7.82-7.84 (4H, m), 7.08 (2H, d), 6.67 (2H, d), 6.67 (2H, d), 5.12 (2H, d), 4.14 (2H, s), 4.11 (2H, m), 3.93 (2H, t), 3.57-3.63 (8H,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com