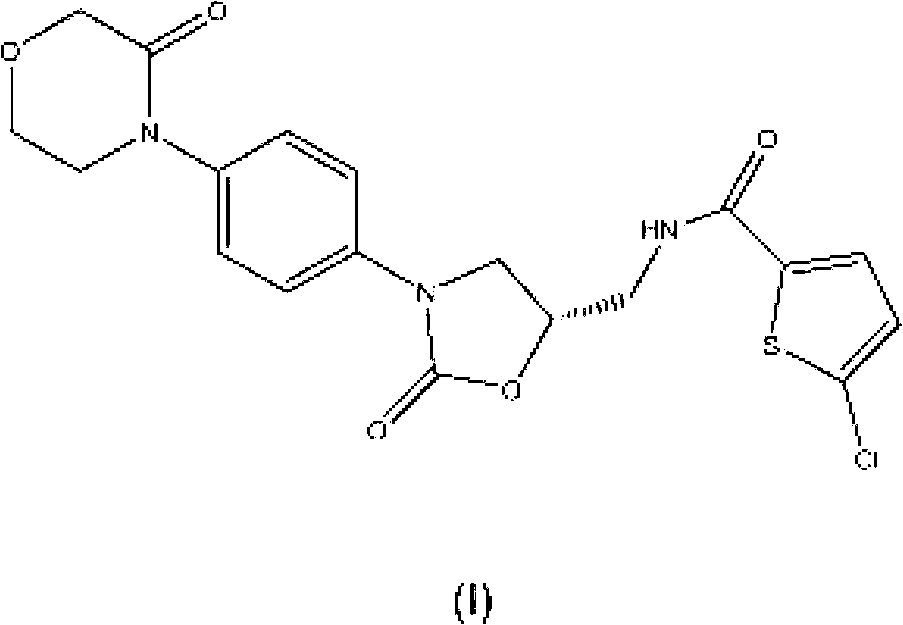
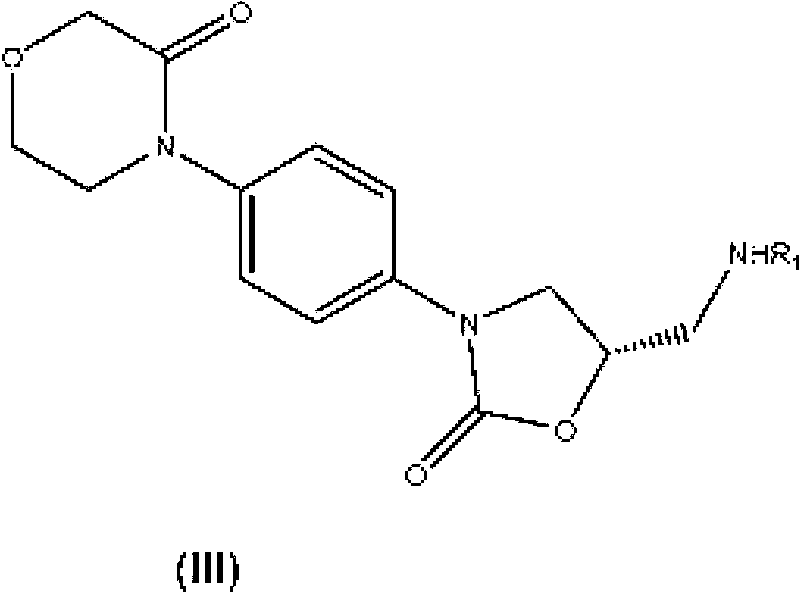
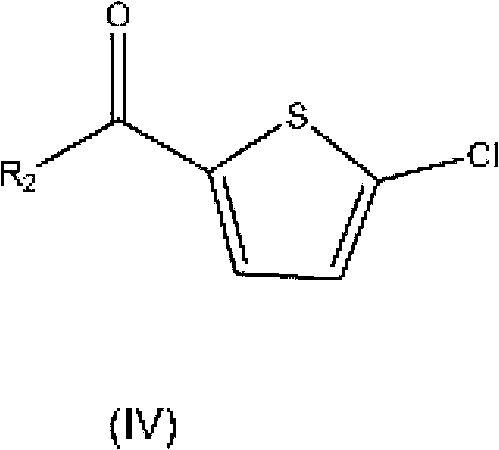
Process for the preparation of rivaroxaban and intermediates thereof
A technology of rivaroxaban and compounds, applied in organic chemistry and other directions, can solve problems such as danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1: Preparation of 4-phenylmorpholin-3-ketone
[0085]
[0086] To a solution of 2-aniline ethanol (6.0 mL, 47.8 mmol) in IPA (6 mL) heated to 40° C. was simultaneously added dropwise chloroacetyl chloride (11.4 mL, 143.4 mmol) and 10 N NaOH (29.6 mL, 296 mmol), keeping A pH of about 7-8. After the addition, the mixture was stirred at 40° C. for 10 min, cooled to 0° C. and stirred at this temperature for 1 hour. The white solid was collected by filtration, washed with cold water and dried to give 4-phenylmorpholin-3-one (5.27 g, 62%) as a white solid. 1 H NMR (400MHz, CDCl 3 )δ7.42(t, J=8.0Hz, 2H), 7.34-7.27(m, 3H), 4.35(s, 2H), 4.04(t, J=5.2Hz, 2H), 3.77(t, J=5.2 Hz, 2H).
Embodiment 2
[0087] Embodiment 2: Preparation of 4-(4-nitrophenyl) morpholin-3-one (compound XI)
[0088]
[0089] To 4-phenylmorpholin-3-one (19.9 g, 0.11 moles) cooled to -10 ° C in 95% H 2 SO 4 (46.6 mL) was carefully added dropwise with 65% HNO over 45 minutes 3 (8 mL). The obtained red solution was stirred at this temperature for 1 h and H 2 O (110 mL). The mixture was washed with 35% NH 4 OH is neutralized to pH=7. The resulting precipitate was filtered, washed with cold water and dried to yield 4-(4-nitrophenyl)morpholin-3-one (or compound XI) as a brown solid (20 g, 83%). 1 H NMR (200MHz, CDCl 3 )δ8.27(d,J=9.4Hz,2H),7.62(d,J=9.4Hz,2H),4.38(s,2H),4.08(t,J=5.0Hz,2H),3.85(t, J=5.0Hz, 2H).
Embodiment 3
[0090] Embodiment 3: Preparation 4-(4-aminophenyl) morpholin-3-ketone (compound X)
[0091]
[0092] To a THF solution (200 mL) of 4-(4-nitrophenyl)morpholin-3-one (19.9 g, 89.5 mmol) was added Pd / C (5%) (1.7 g, water content: 58%). H 2 Filled, the mixture was stirred at 70 °C and 3 bar pressure for 6.5 h. Upon completion of the reaction (by HPLC-MS), more Pd / C (900 mg) was added and the mixture was stirred under the same reaction conditions until all starting material was consumed. The catalyst was filtered through celite, and the filtrate was concentrated to give 4-(4-aminophenyl)morpholine-3 (or compound X) (15.3 g, 89%) as a brown solid. 1 H NMR (400MHz, CDCl 3 )δ7.07(d,J=8.8Hz,2H),6.69(d,J=8.8Hz,2H),4.32(s,2H),4.00(t,J=4.8Hz,2H),3.72(bs, 2H), 3.69 (t, J=4.8Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com