Synthesis method of rivaroxaban
A technology of rivaroxaban and a synthetic method, which is applied in the field of synthesis of small-molecule drug rivaroxaban, can solve problems such as difficult removal of by-products, low synthesis yield, and unsuitable synthetic routes for industrial scale-up production, etc. Production cost, simple reaction conditions, and the effect of improving the total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
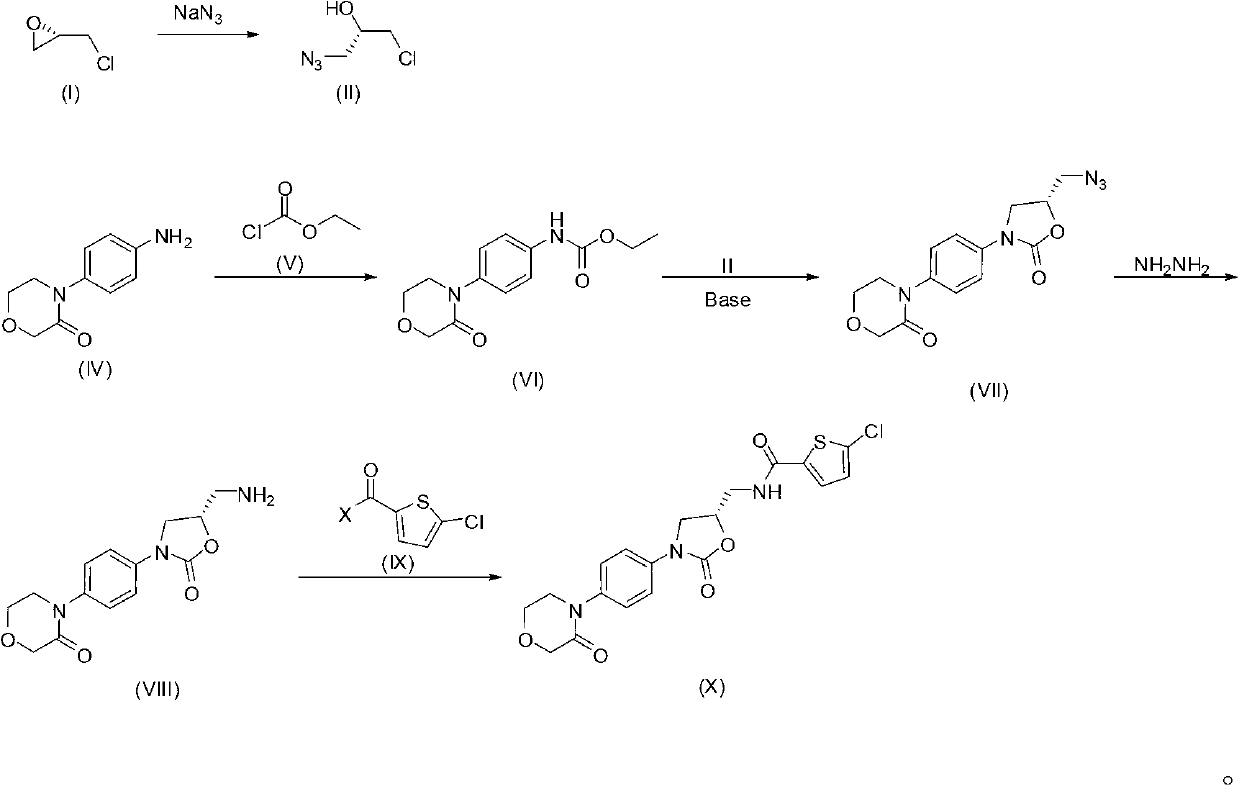
[0047] Synthesis of the compound of embodiment 1 formula (II)
[0048]
[0049]Dissolve sodium azide (3.00 g, 46.1 mmol) and compound of formula (I) (1.00 mg, 10.8 mmol) in water (10 mL), cool to 0 °C, add acetic acid (5 mL), and stir at 0 °C After 1 hour, rise to room temperature and stir overnight; after the reaction is complete, extract three times with dichloromethane, wash the organic phase twice with saturated aqueous sodium bicarbonate, dry over anhydrous sodium sulfate, filter and concentrate to the compound of formula (II) (1.20 g, the yield was 83%). Repeat the reaction steps in this example to prepare more compound of formula (II) for use in subsequent examples.
[0050] 1 H-NMR (CDCl 3 , 400MHz, δppm): 2.81(d, 1H, J=5.6Hz), 3.41(d, 2H, J=5.6Hz), 3.54(m, 2H), 3.92(m, 1H).
Embodiment 2
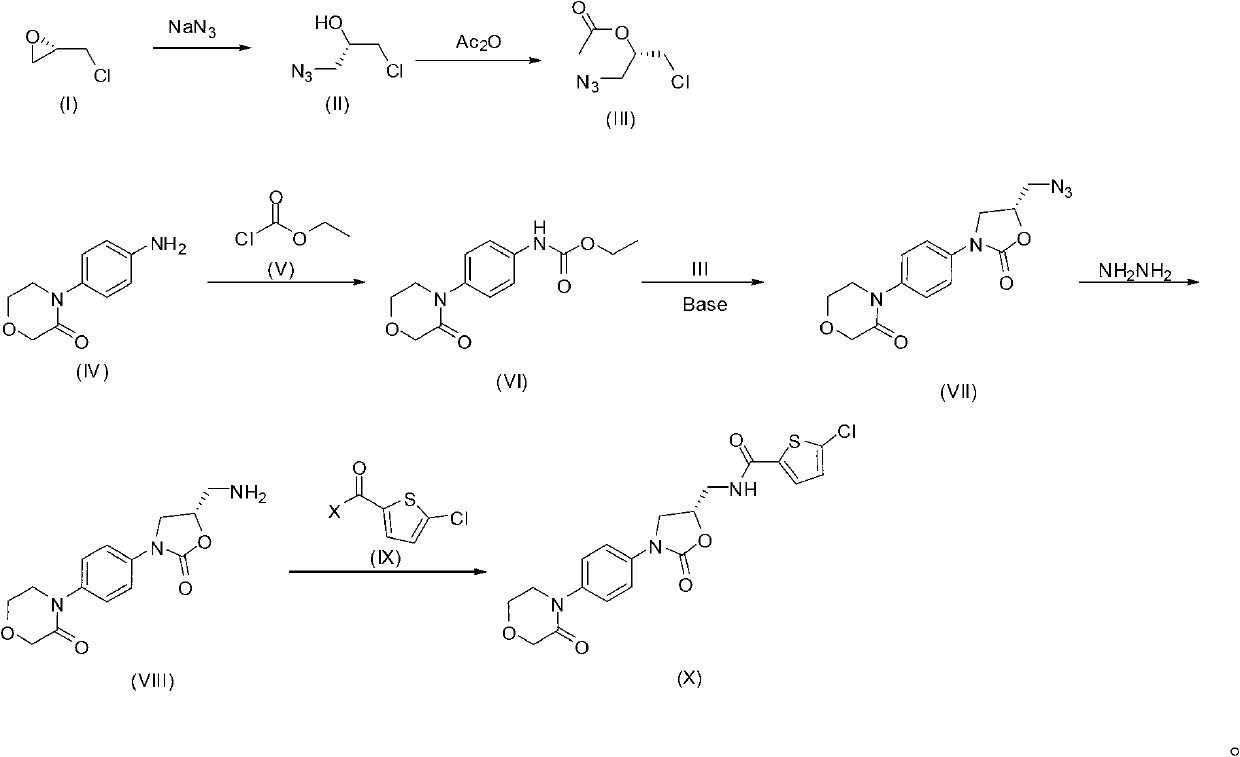
[0051] Synthesis of the compound of embodiment 2 formula (III)
[0052]
[0053] Dissolve the compound of formula (II) (2g, 14.8mmol) and acetic anhydride (3g, 29.6mmol) in dichloromethane (30mL), heat to 30°C, add pyridine (2.34g, 29.6mmol), and stir at the same temperature overnight; after the reaction was complete, it was concentrated and subjected to column chromatography (petroleum ether: ethyl acetate = 30:1 to 10:1) to obtain the compound of formula (III) (1.5 g, yield 58%).
[0054] 1 H-NMR (CDCl 3 , 400MHz, δppm): 2.12(s, 3H), 3.56(m, 2H), 3.66(m, 2H), 5.10(m, 1H).
Embodiment 3
[0055] Synthesis of the compound of embodiment 3 formula (VI)
[0056]
[0057] Dissolve the compound of formula (IV) (500mg, 2.60mmol) in acetone (10mL), add sodium bicarbonate (241mg, 2.86mmol) and water (13mL), cool to 0°C, add dropwise the compound of formula (V) The compound (299 mg, 2.76 mmol) was stirred overnight; after the reaction, water (20 mL) was added, filtered, washed with water, and dried to obtain the compound of formula (VI) (637 mg, yield 93%).
[0058] 1 H-NMR (CDCl 3 , 400MHz, δppm): 1.31(t, 3H, J=7.2Hz), 3.73(t, 2H, J=5.2Hz), 4.02(t, 2H, J=5.2Hz), 4.22(dd, 2H), 4.33 (s, 2H), 6.79 (s, 1H), 7.25 (d, 2H, J=3.2Hz), 7.40 (d, 2H, J=4.8Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com