Method for synthesizing rivaroxaban
A synthesis method and technology of rivaroxaban, applied in the direction of organic chemistry and the like, can solve the problems of many steps, low yield and high requirements for operating conditions, and achieve the effects of low cost, high yield and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
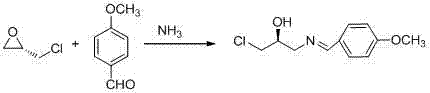
[0044] Preparation of compound Ⅰ (S)-1-chloro-3-[(4-chloro-E-benzylidene)-amino]-propan-2-ol
[0045]
[0046] Method A
[0047] Dissolve 20.0 g (142.9 mmol) of p-chlorobenzaldehyde in 80 ml of anhydrous methanol, add 13.7 ml (191.8 mmol of ammonia) of concentrated ammonia water, stir for 20 min, then add dropwise 13.3 g (143.8 mmol) of (S)-(+) -Epichlorohydrin, reacted at 20°C for 12 h; then raised the temperature to 40°C for 2 h; TLC monitored the complete reaction of p-chlorobenzaldehyde, concentrated under reduced pressure, recrystallized from toluene and n-heptane, and obtained 1.28 g of white snowflake-like crystals, obtained The rate is 85%.
[0048] Method B
[0049] Dissolve 1.12 g (7.80 mmol) of p-chlorobenzaldehyde in 5 ml of anhydrous methanol, add 1 ml (containing 20.00 mmol of ammonia) ammonia saturated methanol solution, stir for 20 min, then add dropwise 0.66 g (7.20 mmol) (S) -(+)-Epichlorohydrin, reacted at 20°C for 12 h; then raised the temperature to...
reference example 2
[0052] Preparation of compound Ⅰ (S)-1-chloro-3-[(4-methoxy-E-benzylidene)-amino]-propan-2-ol
[0053]
[0054] Dissolve 1.36 g (10.0 mmol) of 4-methoxybenzaldehyde in 5 ml of anhydrous methanol, add 1 ml (containing 20.00 mmol of NH 3 ) ammonia saturated methanol solution, stirred for 20 min, then added dropwise 0.92 g (10.0 mmol) (S)-(+)-epichlorohydrin, reacted at 20 °C for 12 h, then raised the temperature to 40 °C for 2 h; TLC monitored 4- Methoxybenzaldehyde was completely reacted, concentrated under reduced pressure, and recrystallized from toluene and n-heptane to obtain 2.08 g of white snowflake crystals with a yield of 92%.
[0055]1 H-NMR (CDCl 3 ) δ: 3.69 (2 H, bs), 3.80 (5 H, m), 4.15 (1 H, s), 7.41 (2 H, d, J =8.0 Hz), 7.69 (2 H, d, J =8.0 Hz), 8.33 (1 H, s)
Embodiment 1
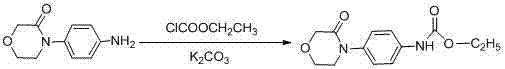
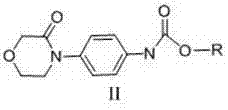
[0057] Preparation of compound Ⅱ 4-(morpholin-3-onephenyl)-carbamate methyl ester
[0058]
[0059] Dissolve 0.3 g (1.56 mmol) 4-(4-aminophenyl)-3-morpholinone in 5 ml ethyl acetate, then add 0.36 g (4.29 mmol) NaHCO 3 and 2 ml of water, cooled to 0°C, added dropwise 0.15 ml (1.88 mmol) of methyl chloroformate and 0.8 ml of chloroform, gradually warmed to room temperature, and then continued to react for 1 h, TLC monitored the reaction was complete; then added 10 ml of acetic acid Extracted with ethyl ester, washed the organic layer with water, concentrated under reduced pressure, and dried in vacuo at 40°C to obtain 0.39 g of white solid with a yield of 100%.
[0060] 1 H-NMR (CDCl 3 ) δ: 3.73 (2 H, m), 3.78 (3 H, s), 4.03 (2 H, m), 4.33 (2 H, s), 6.69 (1 H, s), 7.27 (2 H, d, J =9.0 Hz), 7.42 (2 H, d, J =8.0Hz)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com