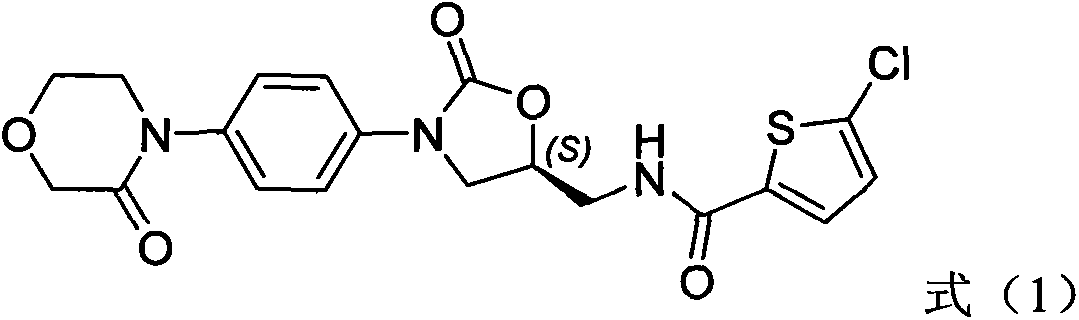
Medicinal composition containing rivaroxaban and preparation method thereof
A technology of rivaroxaban and composition, applied in the field of oral solid pharmaceutical composition, capable of solving the problems of slow dissolution of rivaroxaban and complex preparation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
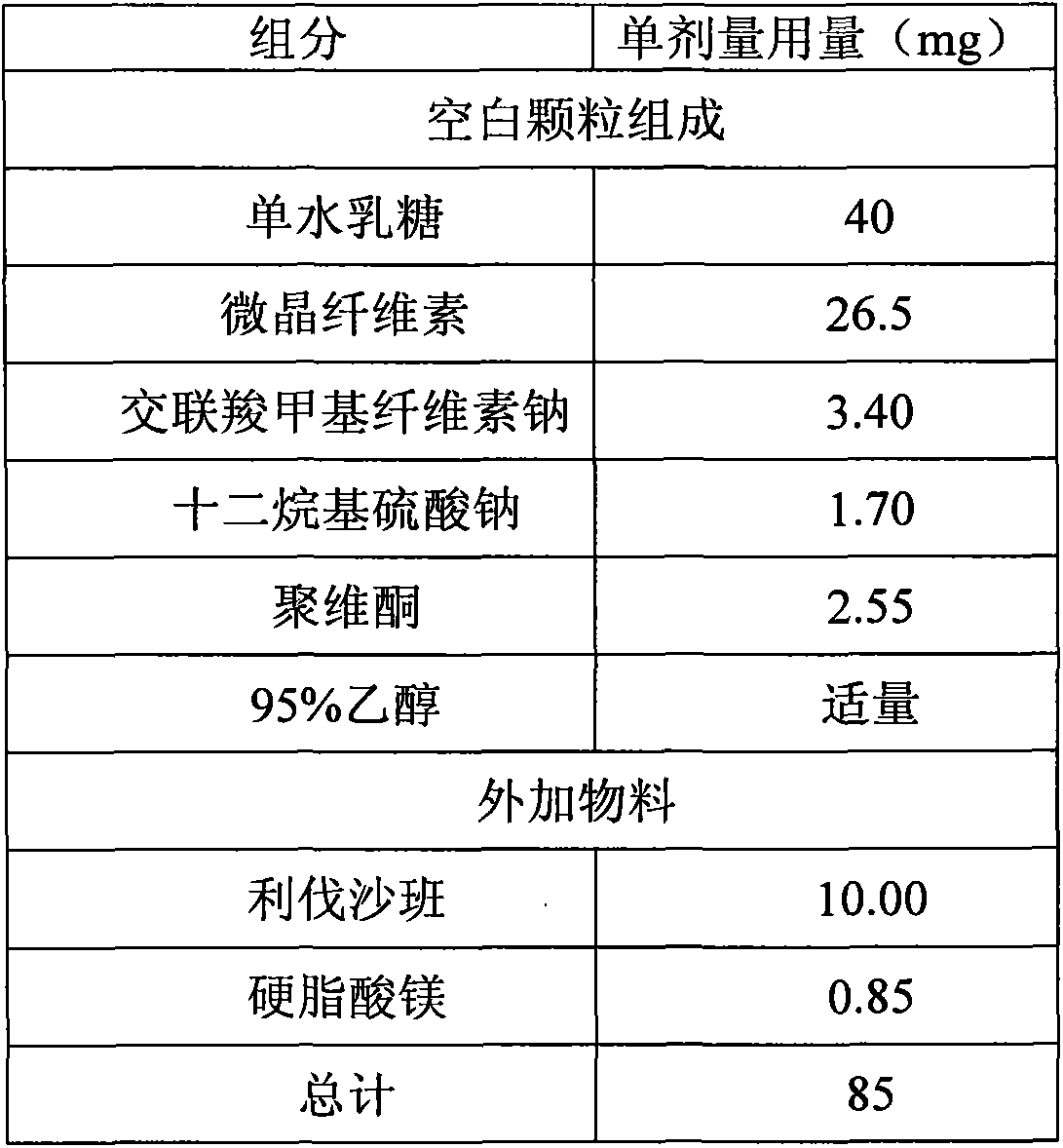
[0033] The prescription composition was the same as that of Comparative Example 1. The lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, and sodium lauryl sulfate were mixed uniformly in a high-shear wet granulator, and a binder solution was added. Go through a 2.0mm sieve and sizing, and dry in a fluidized bed until the loss on drying (LOD) is less than 3.0%. The dry granules are sized through a 1.2mm sieve, then add the micronized rivaroxaban and mix well, then add the hard Magnesium fatty acid blend. The mixed granules are compressed into tablets with a diameter of 6mm and a hardness of 2-6kp.
[0034] The tablet weight, hardness, disintegration time limit, and friability of the samples of Comparative Examples 1 to 2 and Example 1 were tested, and the results were as follows:
[0035] Sample
Average tablet weight / mg
Average hardness / kp
Disintegration time limit
Comparative Example 1
85.4
4.5
15s
0.19%
Comparative Example 2
85.0
4.9 ...
Embodiment 2
[0040]
[0041] Mix lactose monohydrate, microcrystalline cellulose, croscarmellose sodium and sodium lauryl sulfate in a high-shear granulator, and add povidone in ethanol (95%) solution to granulate. Go through a 2.0mm sieve and sizing, and dry in a fluidized bed until the loss on drying (LOD) is less than 3.0%. The dry granules are sized through a 1.2mm sieve, then add the micronized rivaroxaban and mix well, then add stearic acid Magnesium blend. The mixed granules are compressed into tablets with a diameter of 6mm and a hardness of 2-6kp.
Embodiment 3
[0043]
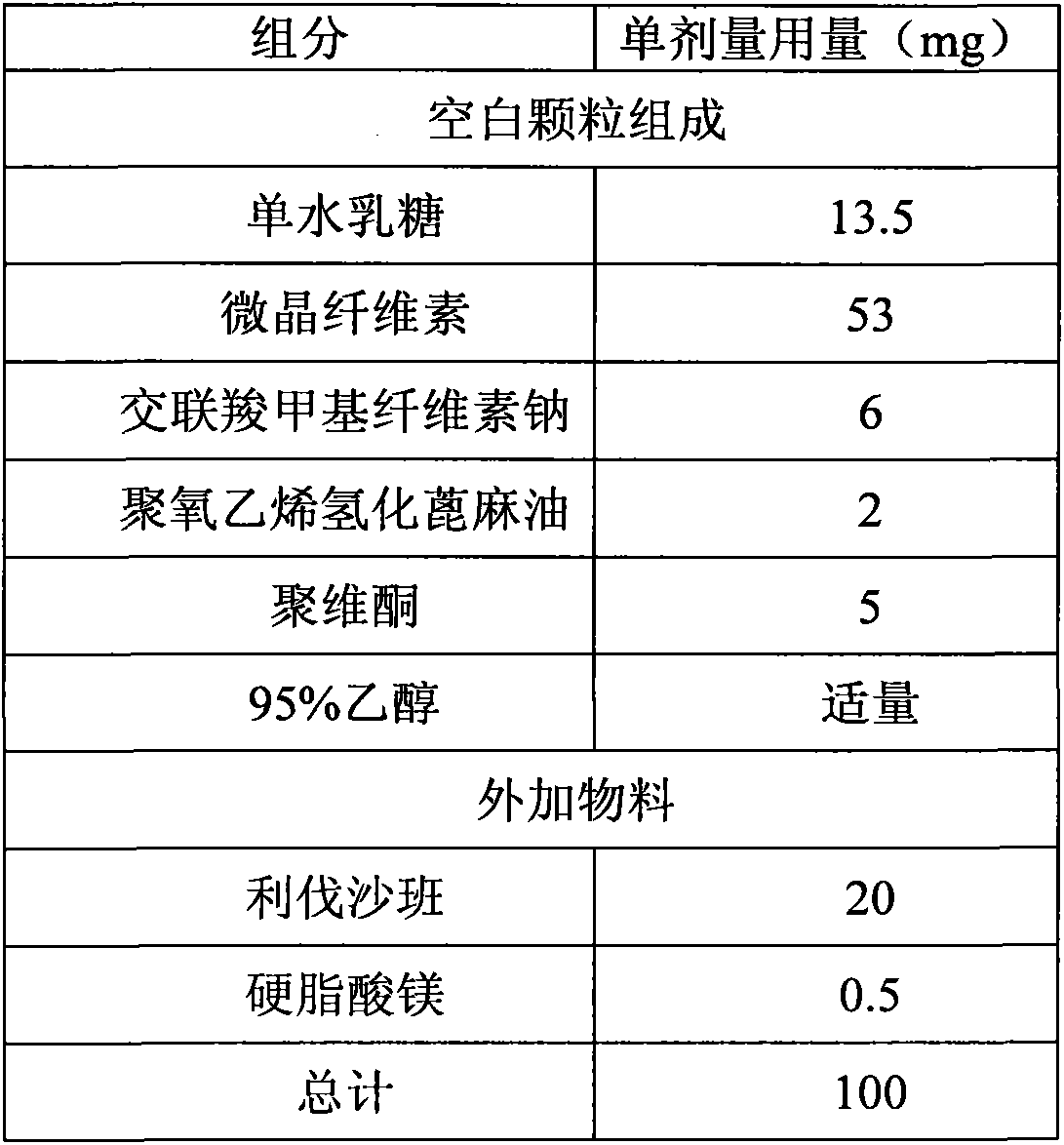
[0044] Povidone is dissolved in 95% ethanol to make a binder solution. The lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, and polyoxyethylene hydrogenated castor oil are placed in a fluidized bed. In the fluidized state, spray the binder solution to granulate, and dry to LOD≤3.0%, pass through a 1.2mm sieve to obtain blank granules, add micronized rivaroxaban and mix well, then add magnesium stearate to mix . The mixed granules are compressed into tablets with a diameter of 6mm and a hardness of 2-6kp.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com