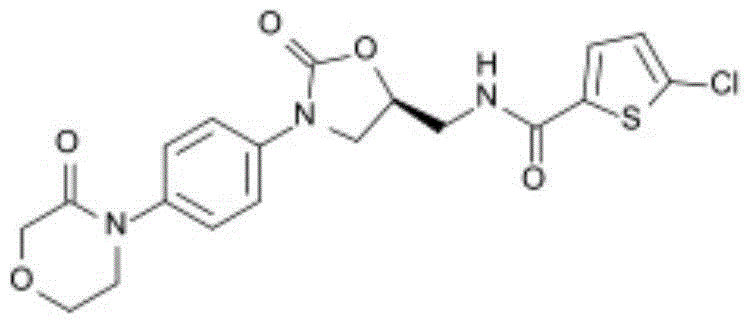
Rivaroxaban pharmaceutical composition and preparation method thereof
A technology of rivaroxaban and composition, applied in the field of medicinal chemistry, can solve the problems of long time, large energy consumption and high cost of wet granulation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] In the second aspect, the composition described in the first aspect of the present invention is prepared by the following method, and the preparation method includes the following steps:
[0027] a) dissolving the solubilizer in the binder solution;
[0028] b) adding rivaroxaban active substance, filler and disintegrant to the granulation equipment, and using a) to obtain the binder solvent to granulate, and dry;
[0029] c) passing the rivaroxaban granules obtained in b) through a comil sieve for granulation, and then placing them in a mixing tank;
[0030] d) Manually mix the lubricant with an appropriate amount of rivaroxaban granules obtained in b), pass through a 30-mesh sieve (0.600mm), add to the mixing tank, and mix to obtain the prepared granules;
[0031] In some embodiments, the preparation method of the present invention further includes the steps of compressing the prepared granules by using a tablet machine and coating the plain tablet after tableting. ...
specific Embodiment approach
[0039] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0040] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0041] Unless otherwise stated, all content percentages given below are mass percentages.
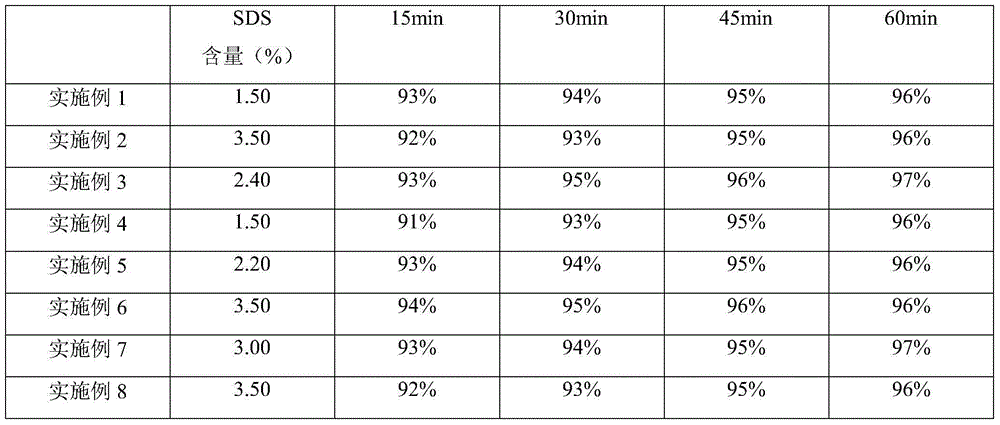
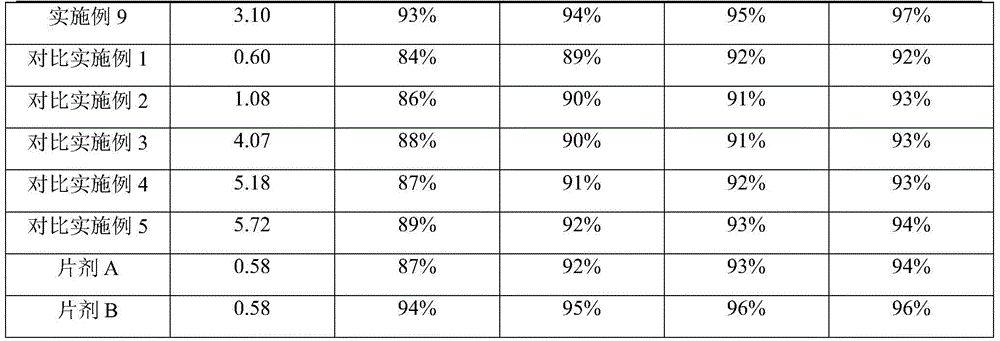
Embodiment 1
[0043] prescription
[0044] name
Content per tablet (mg)
Ratio (%, w / w)
Rivaroxaban, micronized
10.00
10.00
37.80
37.80
[0045] lactose monohydrate
32.50
32.50
7.00
7.00
5.50
5.50
Sodium dodecyl sulfate (SDS)
1.50
1.50
3.00
3.00
Opadry II
2.70
2.70
Total
100.00
100.00
[0046] Preparation:
[0047] Weigh an appropriate amount of sodium dodecyl sulfate (SDS), dissolve it in a hypromellose solution to prepare a binder solution, and set aside. Put rivaroxaban, microcrystalline cellulose, lactose monohydrate and croscarmellose sodium in a fluidized bed, and granulate in the fluidized bed with the previously prepared binder solution, and the fluidized bed The hole diameter of the cloth bag used in the step is less than 20mm, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com