Preparation method of 4-(nitrobenzophenone)-3-morpholone and method for preparing rivaroxaban by using 4-(nitrobenzophenone)-3-morpholone
A technology of nitrophenyl and morpholinone is applied in the field of preparation of rivaroxaban and intermediates thereof, and achieves the effects of saving labor cost, simple operation process and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of 4-(nitrophenyl)-3-morpholinone
[0049] In a 1000mL reaction flask, heat p-chloronitrobenzene (47.1g, 0.3mol) and potassium carbonate (144.9g, 1.05mol) in 500mL of acetonitrile to 60°C, dropwise add ethanolamine (20.2g, 0.33mol) to react 6 Hour. Cool down to 0°C, add chloroacetyl chloride (37.3g, 0.33mol) dropwise, and react at the same temperature for 2 hours. After the reaction is complete, filter, and concentrate the filtrate to dryness under reduced pressure, add 200mL water and 200mL ethyl acetate, extract and separate layers, wash the organic layer with 100mL saturated brine, concentrate under reduced pressure to dryness, and refine with ethanol to obtain 4-(nitrate phenyl)-3-morpholinone (57.9 g), yield 87%, purity 99.5%.
[0050] 1H-NMR (CDCl3, 300MHz): δ8.31(d, 2H), 6.86(d, 2H), 4.31(s, 1H), 3.52–3.55(m, 3H)
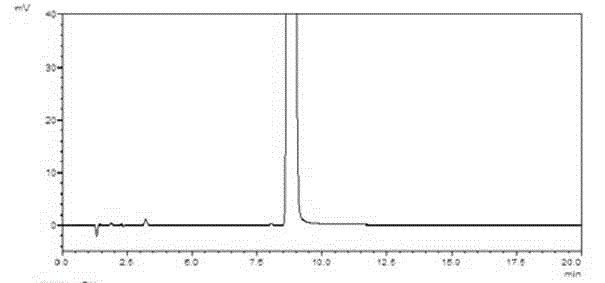
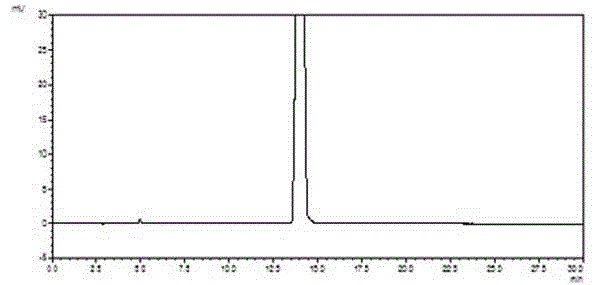
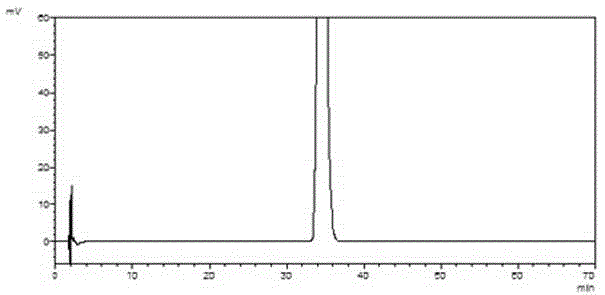
[0051] The chromatographic conditions are:
[0052] Column: Huapu C18, 4.6×250mm, 5μm
[0053]Mobile phase: acetonitril...
Embodiment 2
[0057] Example 2: Preparation of 4-(nitrophenyl)-3-morpholinone
[0058] In a 1000mL reaction flask, p-fluoronitrobenzene (42.3g, 0.3mol) and potassium carbonate (144.9g, 1.05mol) were heated to 60°C in 500mL of acetonitrile, and ethanolamine (20.2g, 0.33mol) was added dropwise to react 18 Hour. Cool down to 0°C, add chloroacetyl chloride (37.3g, 0.33mol) dropwise, and react at the same temperature for 2 hours. After the reaction is complete, filter, and concentrate the filtrate to dryness under reduced pressure, add 200mL water and 200mL ethyl acetate, extract and separate layers, wash the organic layer with 100mL saturated brine, concentrate under reduced pressure to dryness, and refine with ethanol to obtain 4-(nitrate phenyl)-3-morpholinone (53.3 g), yield 80%, purity 99.5%.
Embodiment 3
[0059] Embodiment 3: Preparation of 4-(nitrophenyl)-3-morpholinone
[0060] In a 1000mL reaction flask, p-bromonitrobenzene (60.6g, 0.3mol) and potassium carbonate (144.9g, 1.05mol) were heated to 60°C in 500mL of acetonitrile, and ethanolamine (20.2g, 0.33mol) was added dropwise to react 4 Hour. Cool down to 0°C, add chloroacetyl chloride (37.3g, 0.33mol) dropwise, and react at the same temperature for 2 hours. After the reaction is complete, filter, and concentrate the filtrate to dryness under reduced pressure, add 200mL water and 200mL ethyl acetate, extract and separate layers, wash the organic layer with 100mL saturated brine, concentrate under reduced pressure to dryness, and refine with ethanol to obtain 4-(nitrate phenyl)-3-morpholinone (56.6g), yield 85%, purity 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com