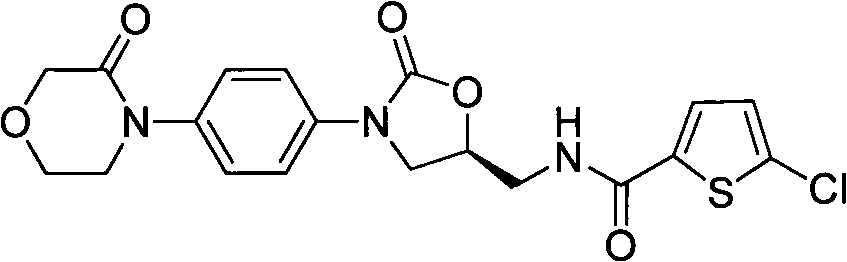
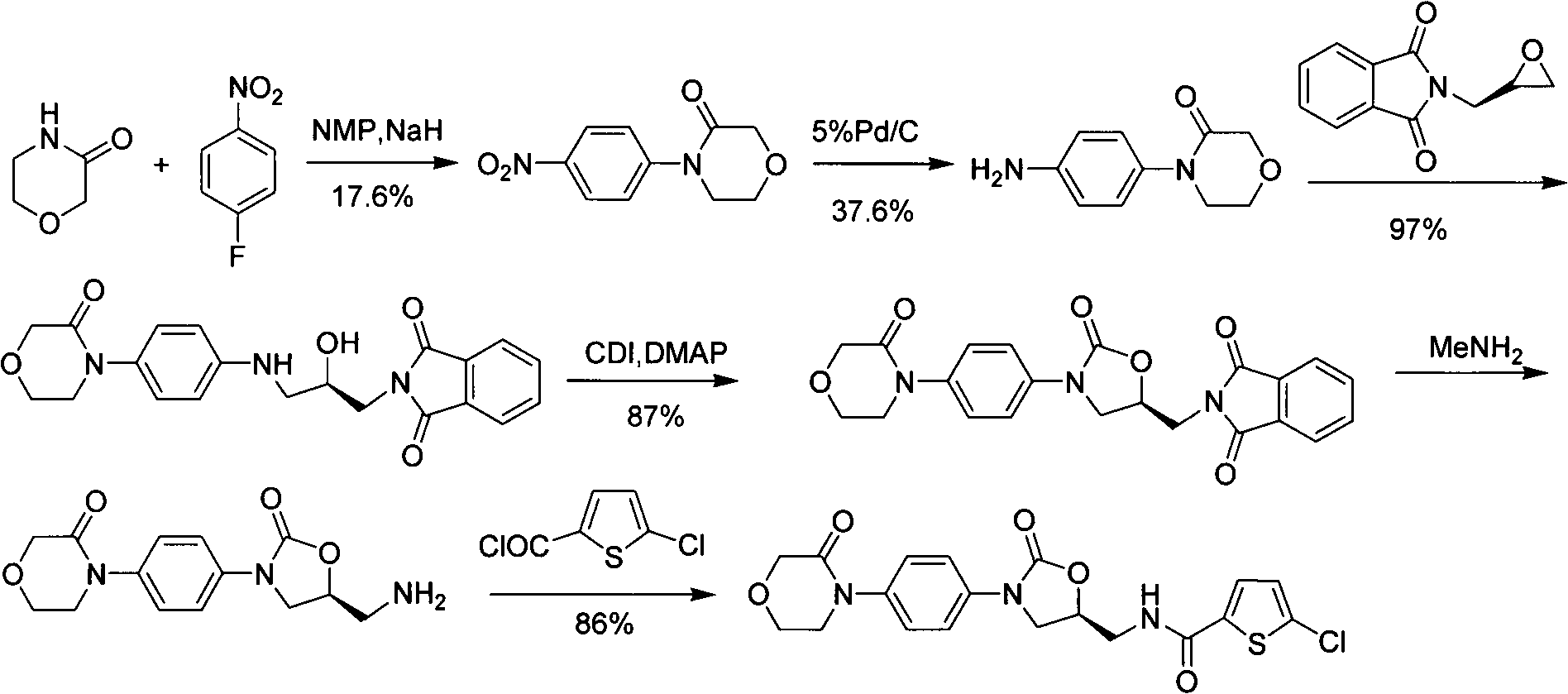
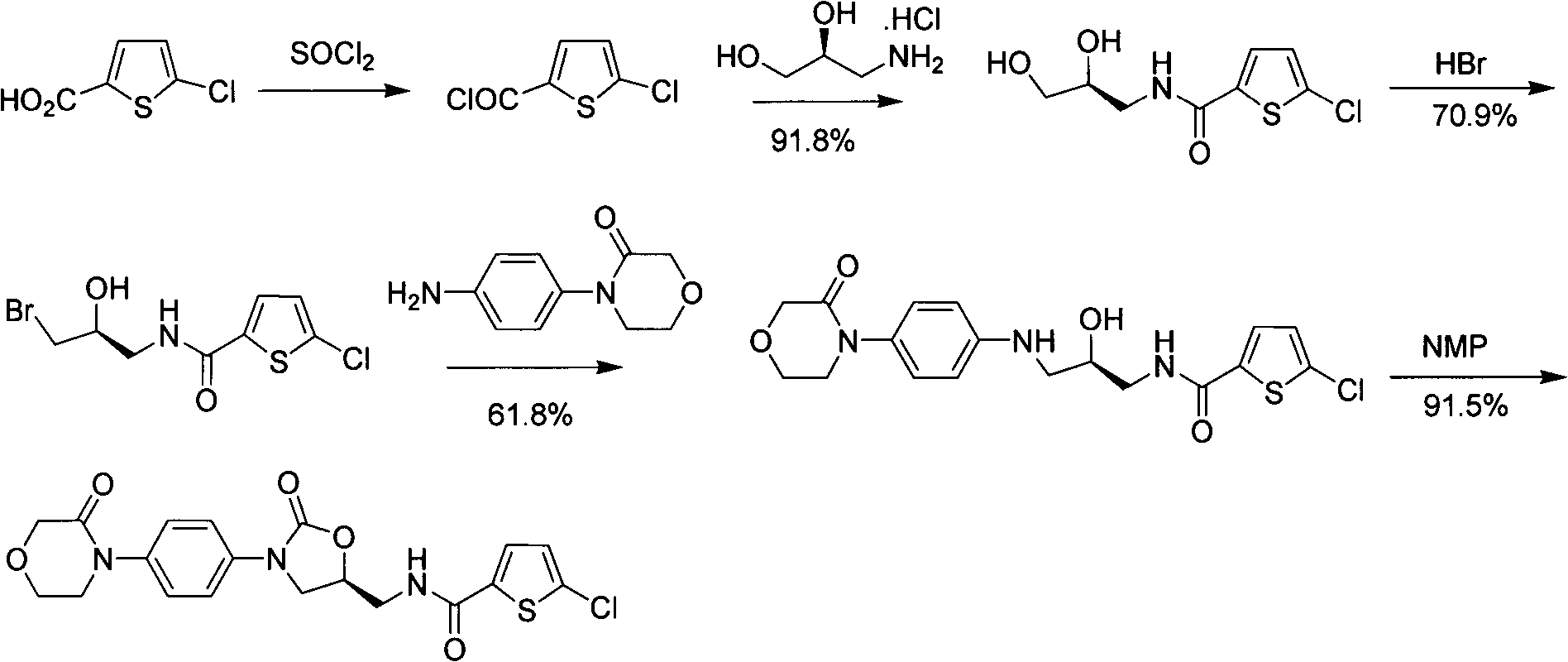
Preparation methods for rivaroxaban and intermediate thereof, and intermediate compounds
A technology for rivaroxaban and compounds is applied in the preparation of rivaroxaban and its intermediates, as well as in the field of intermediate compounds, and can solve problems such as tediousness, potential safety hazards, and unfavorable industrialized production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 (S)-4-(4-(5-((dibenzylamino)methyl)-2-oxooxazolidin-3-yl)phenyl)morpholinyl-3-one (compound 4) Preparation
[0070] tert-butoxycarbonyl-4-(3-oxomorpholinyl)aniline (100.0g, 0.34mol) was added to 500mL DMF, cooled to 0-10°C, and lithium tert-butoxide / THF (410.0g, 1.03mol ), the temperature of the control material is lower than 20°C, and the dripping is completed in half an hour. Add (S)-1-chloro-3-(dibenzylamino)-propan-2-ol (129.0g, 0.45mol) to control the temperature of the feed liquid below 45°C, raise the temperature to 40-50°C, and maintain the reaction for 40 hours until the reaction is complete. After cooling to room temperature, 250 mL of ammonium chloride aqueous solution and 100 mL of methanol were added, the feed solution was cooled to -20°C and stirred for 3 hours, filtered, washed with ice methanol, and dried to obtain 123.1 g of a white solid with a yield of 76.9%.
[0071] 1 H NMR (300MHz, CDCl 3 )δ: 2.86 (m, 2H, CHC H 2 N), 3.51 (m, 2H, N...
Embodiment 2
[0073] Example 2 (S)-4-(4-(5-((dibenzylamino)methyl)-2-oxooxazolidin-3-yl)phenyl)morpholinyl-3-one (compound 4) Preparation
[0074] tert-butoxycarbonyl-4-(3-oxomorpholinyl)aniline (0.29g, 1mmol) was added to 5mL DMF (N,N-dimethylformamide), and the nitrogen gas was exhausted, and the temperature of the feed solution was reduced to -10~ At 0°C, 2.5 mL of lithium tert-butoxide (2.5 mmol) tetrahydrofuran solution was added dropwise, and the mixture was naturally raised to room temperature (25°C) and stirred for 2 hours. (S)-1-Chloro-3-(dibenzylamino)-propan-2-ol (0.32 g, 1.1 mmol) was added, and the reaction was maintained at room temperature for 20 hours until the reaction was complete. Saturated ammonium chloride aqueous solution and ethyl acetate were added, and the layers were separated. The organic layer was washed with water, dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness to obtain 0.32 g of a white solid, with a yield of 67.94%.
[0075] HP...
Embodiment 3
[0076] Example 3 (S)-4-(4-(5-((dibenzylamino)methyl)-2-oxooxazolidin-3-yl)phenyl)morpholinyl-3-one (compound 4) Preparation
[0077] tert-butoxycarbonyl-4-(3-oxomorpholinyl)aniline (0.29g, 1mmol) and lithium tert-butoxide (0.19g, 2.4mmol) were added to 10mL of tetrahydrofuran, exhausted with nitrogen, and stirred at room temperature for 2 hours. (S)-1-Chloro-3-(dibenzylamino)-propan-2-ol (0.32 g, 1.1 mmol) was added, and the feed solution was heated to 60° C. for 8 hours until the reaction was complete. Saturated ammonium chloride aqueous solution and ethyl acetate were added, and the layers were separated. The organic layer was washed with water, dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness to obtain 0.34 g of off-white solid with a yield of 72.18%.
[0078] HPLC: 96.35%. The NMR data is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com