Direct compression polymer tablet core
a technology of polymer and tablet core, applied in the field of polymer materials, can solve the problems of undesirable addition of any material other than the active ingredient, not all therapeutics, and generally inability to achiev
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Characterization of 400 mg and 800 mg Sevelamer Hydrochloride Direct Compression Tablet Cores
Preparation of Tablet Cores
[0023] 400 mg sevelamer hydrochloride tablet cores were prepared from a blend consisting of 5000.0 g sevelamer hydrochloride, 50.0 g colloidal silicon dioxide, NF (Aerosil 200) and 50.0 g stearic acid. The sevelamer hydrochloride was hydrated to moisture content of 6% by weight. The blend was prepared by passing the sevelamer hydrochloride and colloidal silicon dioxide through a #20 mesh screen, transferring the mixture to a 16 quart PK blender and blending for five minutes. The stearic acid was then passed through an oscillator equipped with a #30 mesh screen, transferred into the 16 quart PK blender and blended for five minutes with the sevelamer hydrochloride / colloidal silicon dioxide mixture. The resulting blend was discharged into a drum and weighed. The final blend was then compressed on a 16 station Manesty B3B at 4 tons pressure using 0.2...
example 2
Coating of Sevelamer Hydrochloride Tablet Cores
[0026] Compressed core tablets prepared as described in Example 1 were coated in a coating pan with an aqueous coating solution having a solids composition comprising:
Material% W / WHPMC low viscosity Type 2910, cUSP38.5%HPMCE high viscosity Type 2910, cUSP38.5%diacetylated monoglyceride23.0%
[0027] The coating solution was applied to the compressed cores until a weight gain of approximately 4 to 6% was achieved. Stability studies—controlled room temperature, accelerated conditions, freeze / thaw and photosensitivity—for the coated sevelamer hydrochloride tablets were conducted in accordance with those procedures known in the art and described in the following references: International Committee on Harmonization (ICH) guidance “Q1A-Stability Testing of New Drug Substances and Products” (June 1997); ICH “Q1B-Guidelines for the Photostability Testing of New Drug Substances and Products” (November 1996); and ICH guidance “Q1C-Stability Testi...
example 3
Factors Affecting the Processing and Performance Characteristics of Compressed Tablets (Prior to Coating)
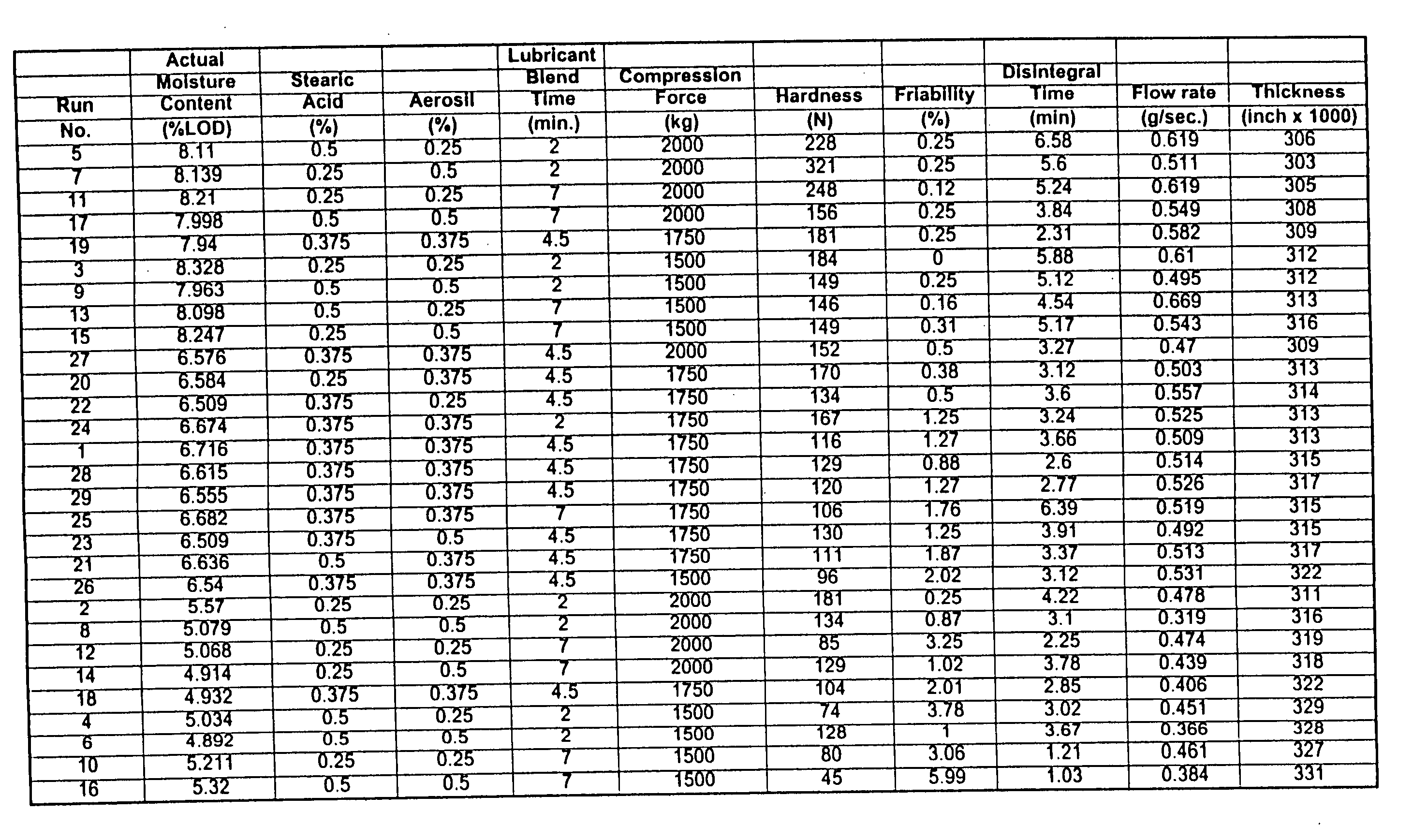
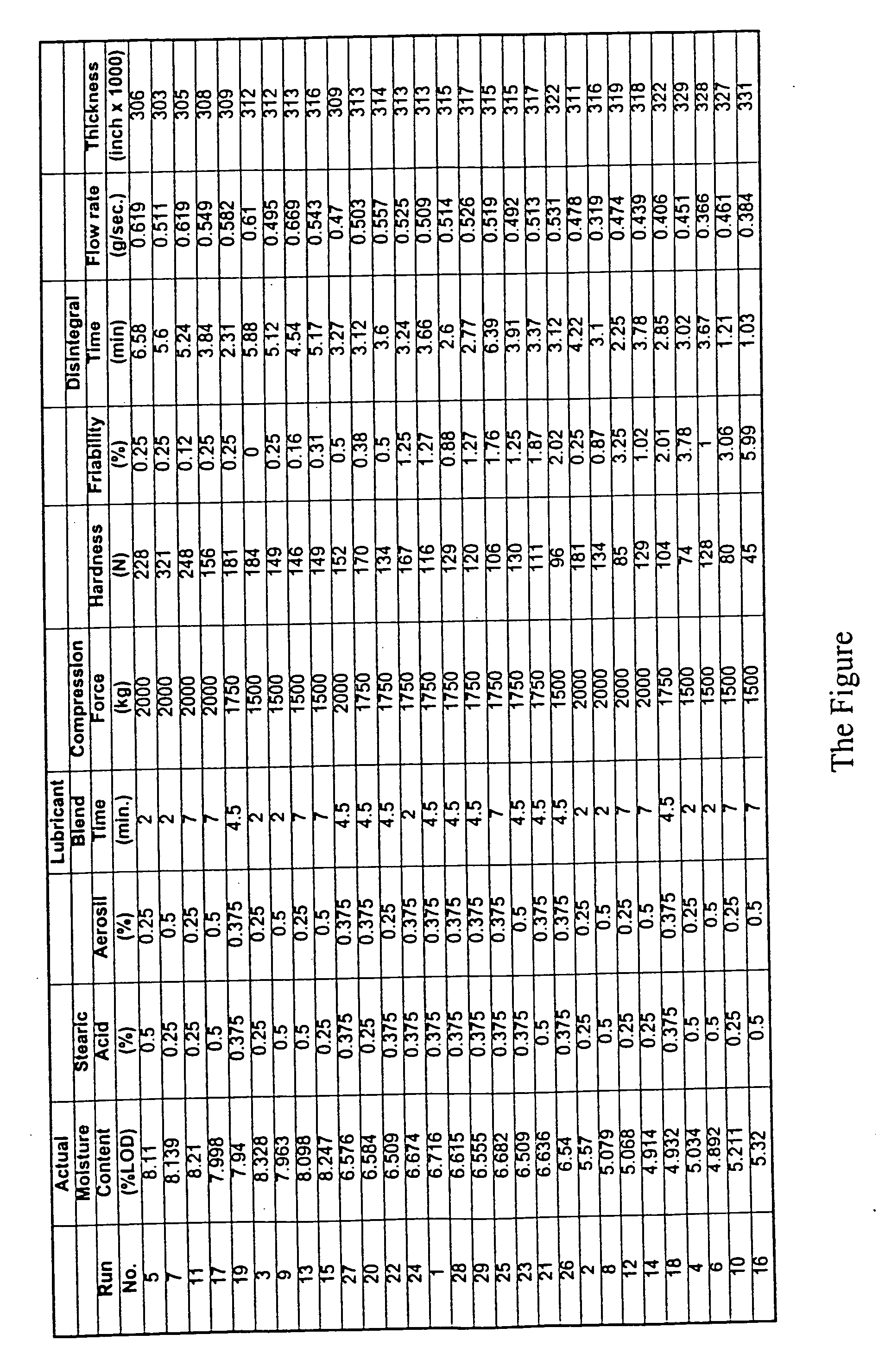
[0028] In order to maintain consistently acceptable compressed tablet on a per batch basis, a number of correlative tests were performed in order to determine which factors most strongly impact the quality and integrity of the tablets. Studies such as weight variation, tablet hardness, friability, thickness, disintegration time, among others are known to those skilled in the art and are described in the United States Pharmacopeia (U.S.P.). “Hardness” means the measure of the force (measured herein in Newtons) needed to fracture a tablet when such tablet is placed lengthwise on a Hardness Tester. “Friability” is the measure of the mechanical strength of the tablet needed to withstand the rolling action of a coating pan and packaging. It is measured using a friabiliator. “Thickness” is the measure of the height of the tablet using a micrometer. “Disintegration Time” is the time ne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com