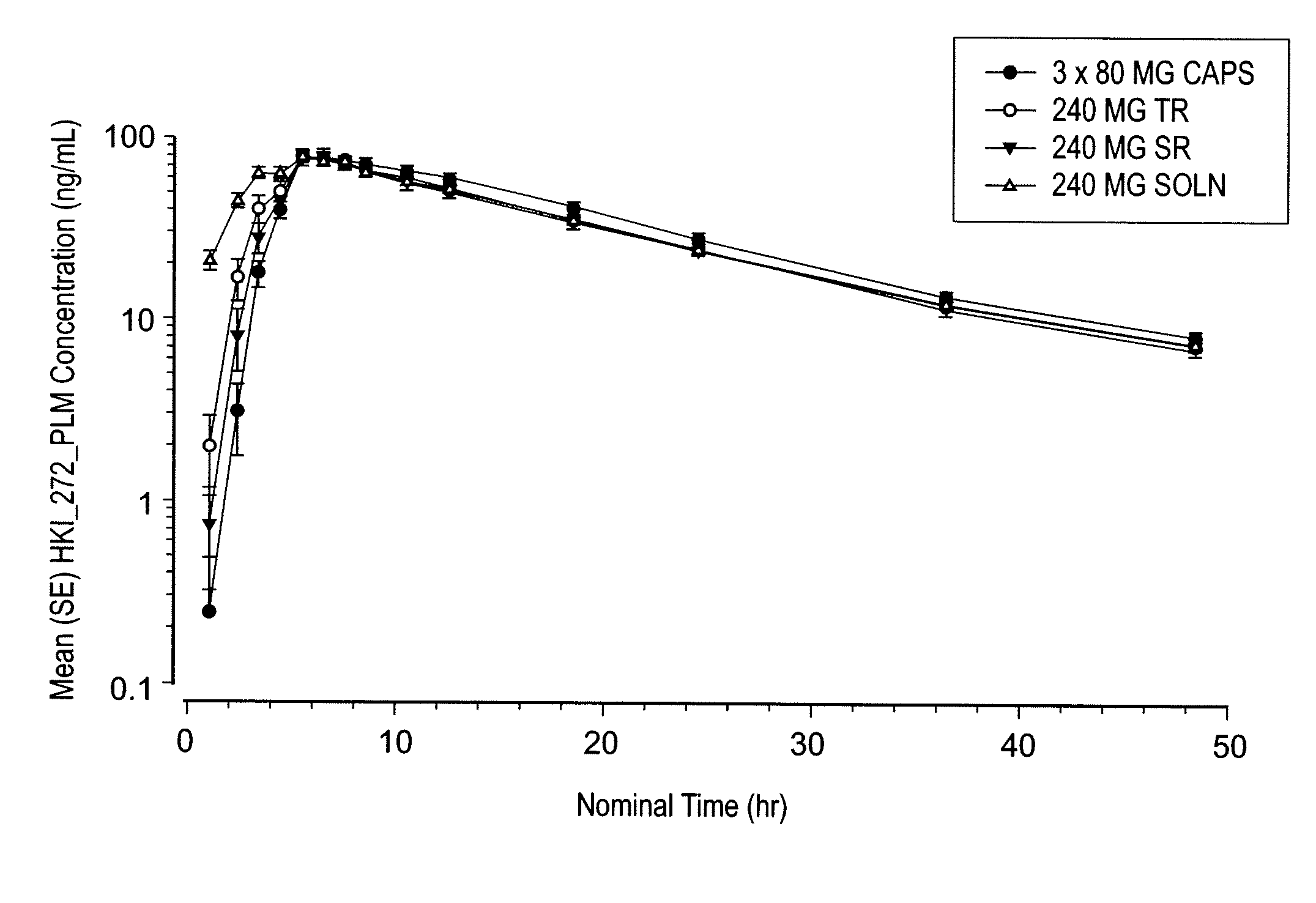
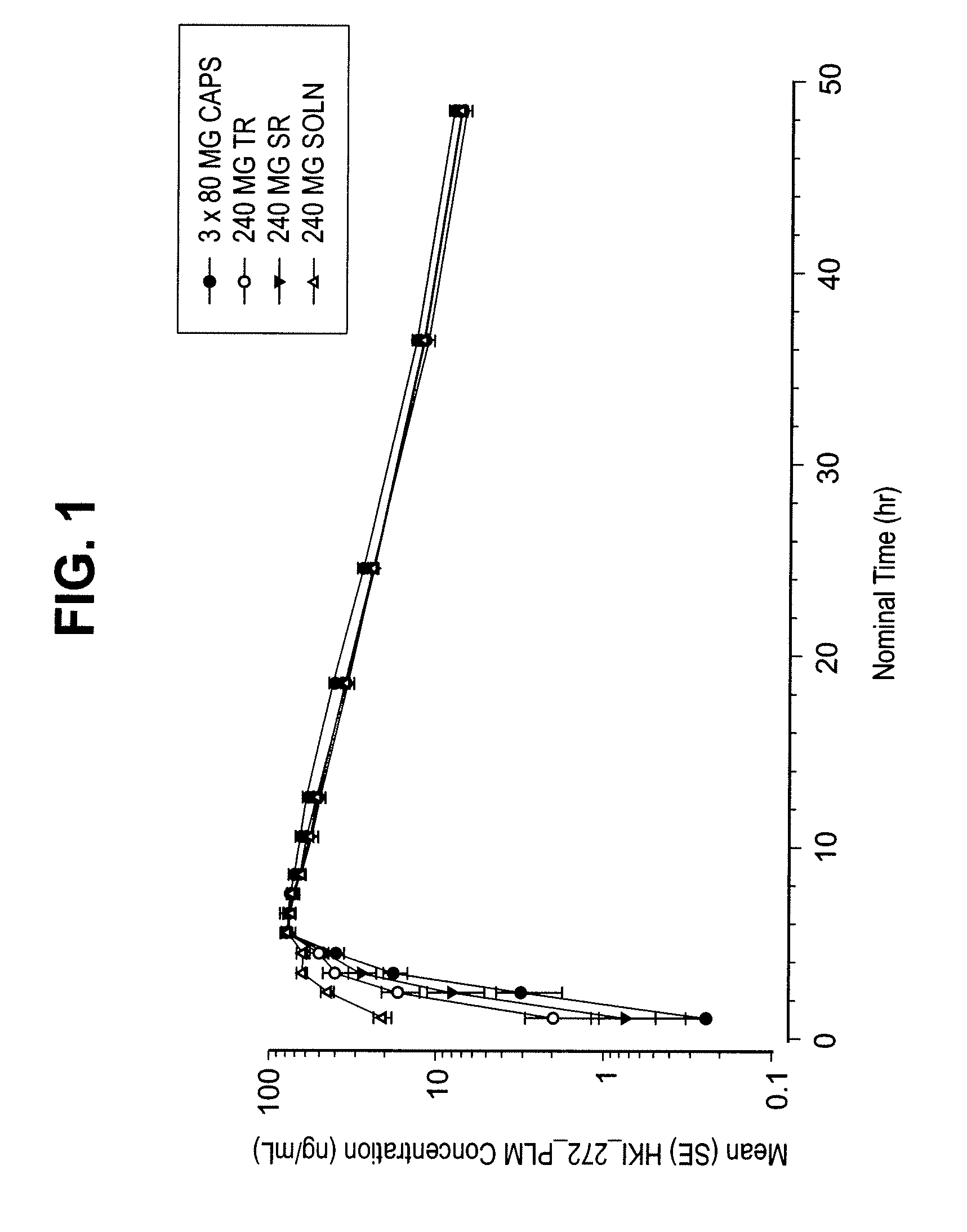
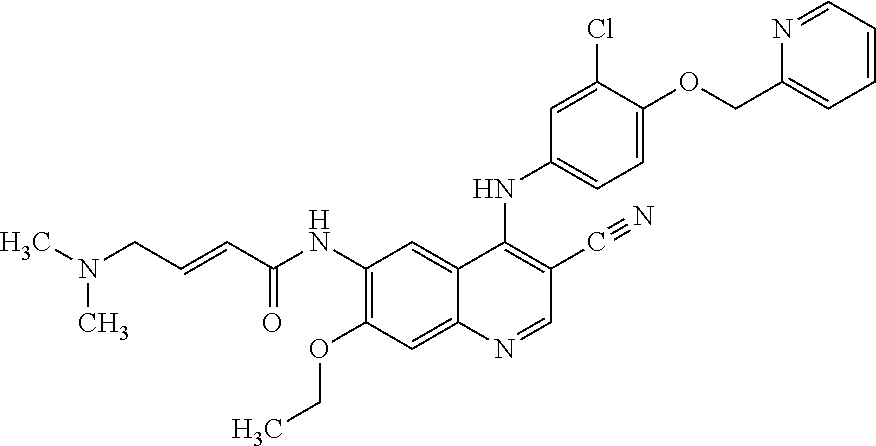
Coated tablet formulations and uses thereof
a technology of coated tablets and formulations, applied in the field of oral pharmaceutical formulations, can solve the problems of chemical degradation and stability, inability to develop a neratinib maleate formulation comprising a capsule or tablet of higher strength, and inability to meet the requirements of pharmaceutical operations, so as to achieve the effect of improving processing characteristics and maintaining acceptable pharmokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Coated Tablets of a Neratinib Maleate Formulation by Fluid Bed Wet Granulation Process
[0041]A pharmaceutically acceptable formulation of neratinib mealate is prepared: a granulation comprising intragranular components (a) 10-70 weight percent of neratinib maleate; (b) 15-65 weight percent of mannitol and microcrystalline cellulose; (c) 0.5-8 weight percent of crospovidone or crosscarmellose sodium; (d) 0.2-8 weight percent of colloidal silicon dioxide, and (e) 5-15 weight percent of povidone. The granulation is combined with extragranular components (f) 4-25 weight percent of microcrystalline cellulose; (g) 1-8 weight percent of crospovidone and (h) 0.5-3 weight percent of magnesium stearate, and then compressed into tablets or dry-filled into capsules. This and certain preferred ranges of materials are shown below in Table 1.
TABLE 1Weight %Weight %ComponentWeight %rangerangeIntra-granular componentsHKI-272 Maleate, anhydrous4120-5010-70Mannitol15-65Microcrystalline c...
example 2
Unit Dosage Forms of an Exemplary Neratinib Maleate Formulation
[0052]Using the fluid bed process described in Example 1, different unit dosages of neratinib maleate were prepared from an exemplary formulation, as summarized in Table 2.
TABLE 2Granulation40 mg80 mg240 mgIngredientFunctionwt / wt (%)mg / tabletMg / tabletmg / tabletIntragranular ComponentsHKI-272 MaleateaActive35.0040.0080.00240.00Mannitol (PearlitolDiluent38.9444.5089.01267.02200 SD)MicrocrystallineDiluent10.5612.0724.1472.41(Avicel PH 101)CrospovidoneDisintegrant3.003.436.8620.57Povidone USP / K-25Surface5.005.7111.4334.29ModifyingAgentColloidal SiO2Glidant2.002.294.5713.71Extragranular ComponentsAvicel PH 101Diluent1.501.713.4310.29CrospovidoneDisintegrant2.002.294.5713.71Mg StearateLubricant2.002.294.5713.71Total Wt.100.00114.29228.57685.71Film Coating:Opadry IIFilm Coat—3.429——(85F15443) Red(3%)Opadry IIFilm Coat——6.86—(85F92177) Yellow(3%)Opadry IIFilm Coat———20.57(85F94211) Pink(3%)Total Tablet Wt—117.714235.43706.28aWeig...
example 3
Coated Tablets of Targeted Release Neratinib Maleate Manufactured by Spraying Povidone on Intragranular Components in a Fluid Bed
[0053]An exemplary targeted release (TR) neratinib maleate formulation is summarized in Table 3.
TABLE 340 mg240 mgTabletTabletIngredient% wt / wt(mg)(mg)FunctionIntragranular ComponentsHKI-272 Maleate, anhydrous35.0040.0240.00ActiveMannitol38.2543.79262.72FillerMicrocrystalline cellulose,12.7514.5086.99FillerCrospovidone3.003.4320.57DisintegrantPovidone5.005.7134.29SurfaceModifyingAgentColloidal Silicon Dioxide0.500.573.43GlidantExtra-granular ComponentsMicrocrystalline cellulose3.003.4320.57FillerCrospovidone2.002.2913.71DisintegrantMagnesium stearate0.500.573.43LubricantTotal (Core Tablet)100.00114.29685.71Film coat:Opadry ®3.4320.57Film coat
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com