Use of the phytocannabinoid cannabidiol (CBD) in combination with a standard Anti-epileptic drug (SAED) in the treatment of epilepsy
a technology of phytocannabinoid cannabidiol and epilepsy, which is applied in the direction of biocide, plant/algae/fungi/lichens, drug compositions, etc., can solve the problems that the selective benefits of cbd with e.g. ethosuximide and valporate (saed's with defined sodium channels) cannot be anticipated, and achieve the effect of reducing the severity and mortality of seizures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
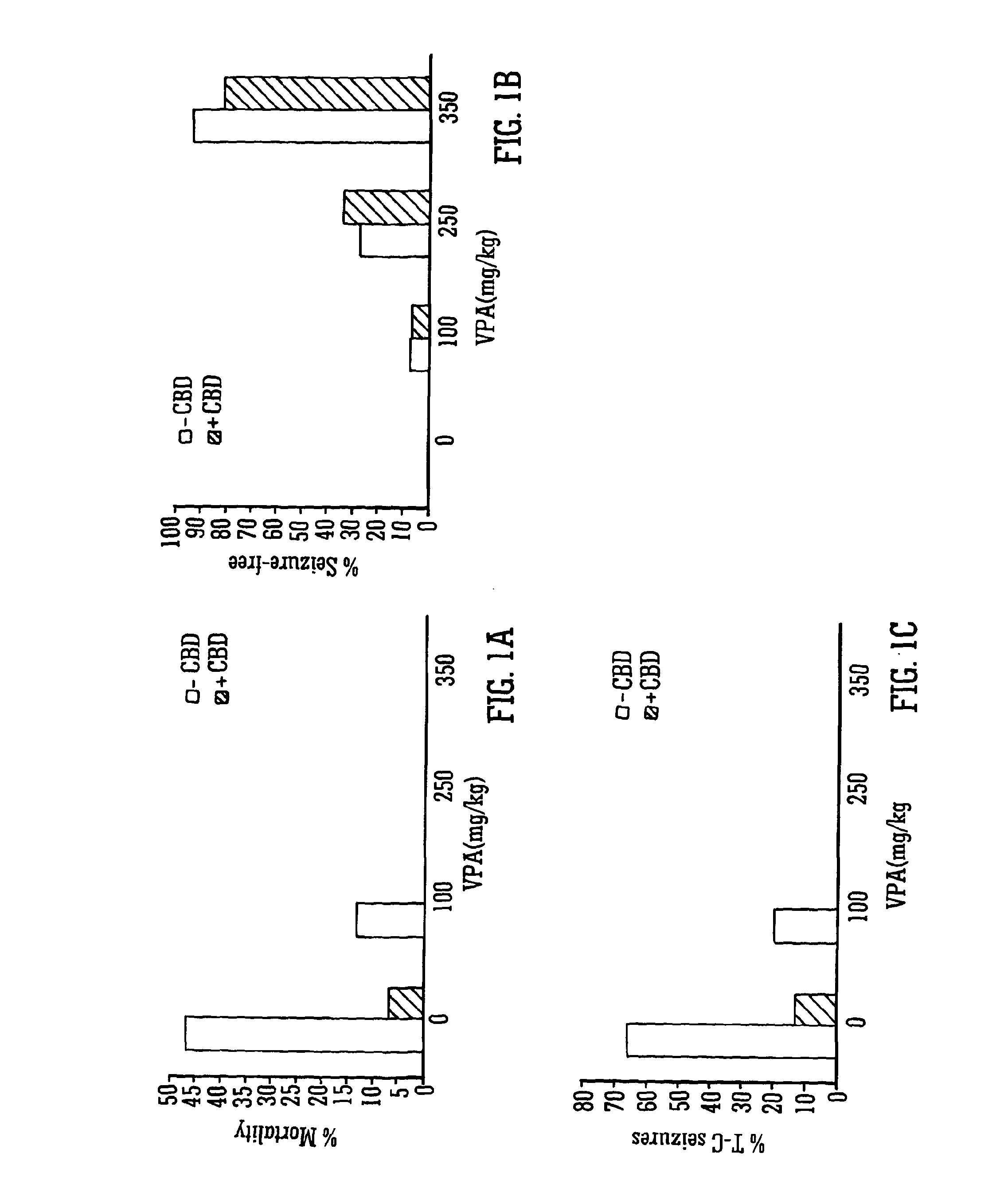
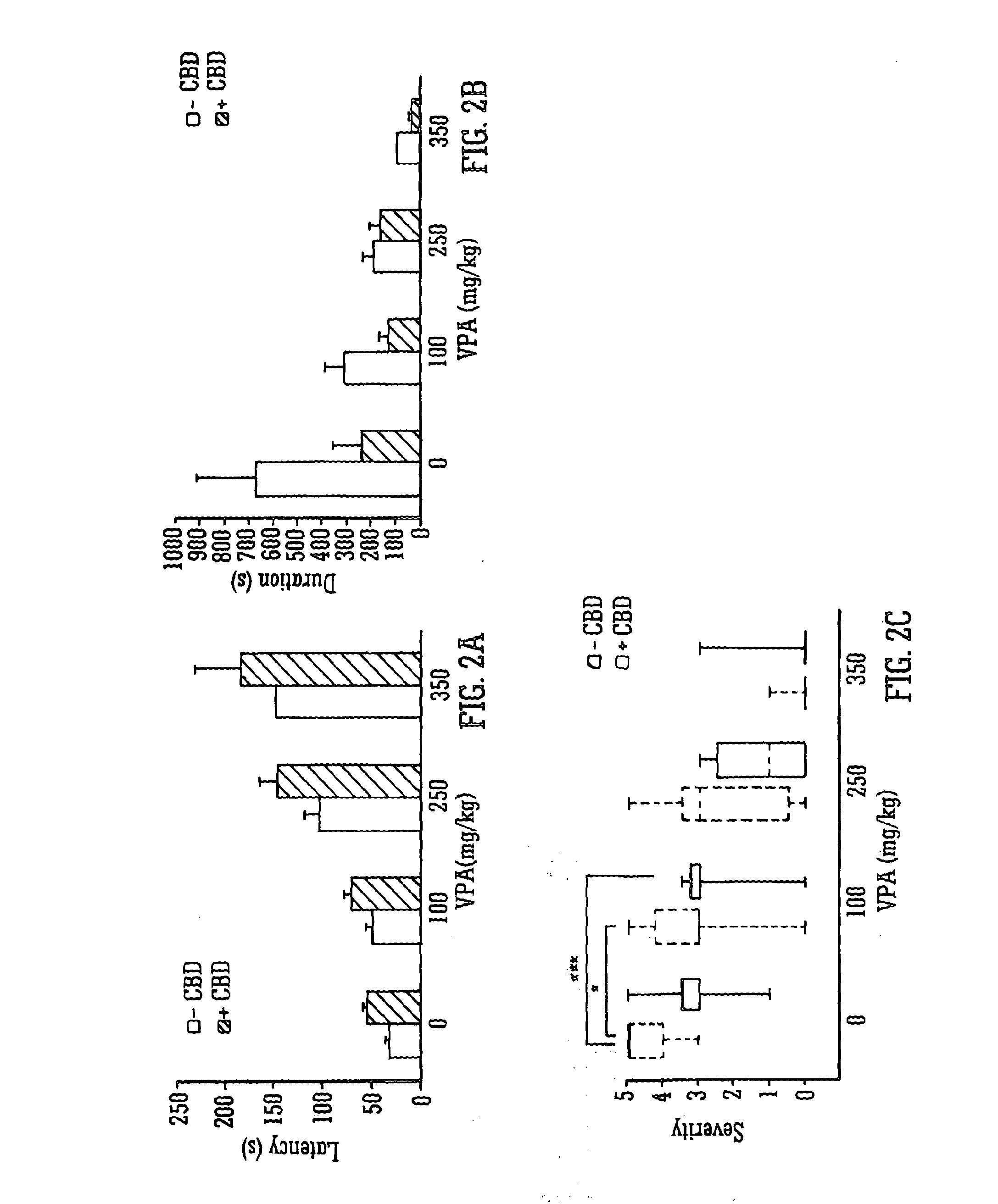
[0070]The Use of the Phytocannabinoid CBD in Combination with a Standard Anti-Epileptic Drug (SAED) in the PTZ-Model of Epilepsy
Methodology:
Animals:
[0071]Male Wistar rats (P24-29; 75-110 g) were used to assess the effects of the phytocannabinoid CBD in combination with SAEDs in the PTZ model of generalised seizures. Animals were habituated to the test environment, cages, injection protocol and handling prior to experimentation. Animals were housed in a room at 21° C. on a 12 hour light: dark cycle (lights on 0900) in 50% humidity, with free access to food and water.
[0072]The human dose equivalent (HED) can be estimated using the following formula:
HED=Animaldose(mg / kg)multipliedbyAnimalKmHumanKm[0073]The Km for a rat is 6 and the Km for a human is 37.
Thus, for a human of approx 60 Kg a 100 mg / Kg dose in rat would equate to a human dose of about 1000 mg. Human doses of greater than 300 mg / day, through 400 mg / day in 100 mg intervals (namely through 500, 600, 700, 800, 900, 1000, 1100, ...
example 2
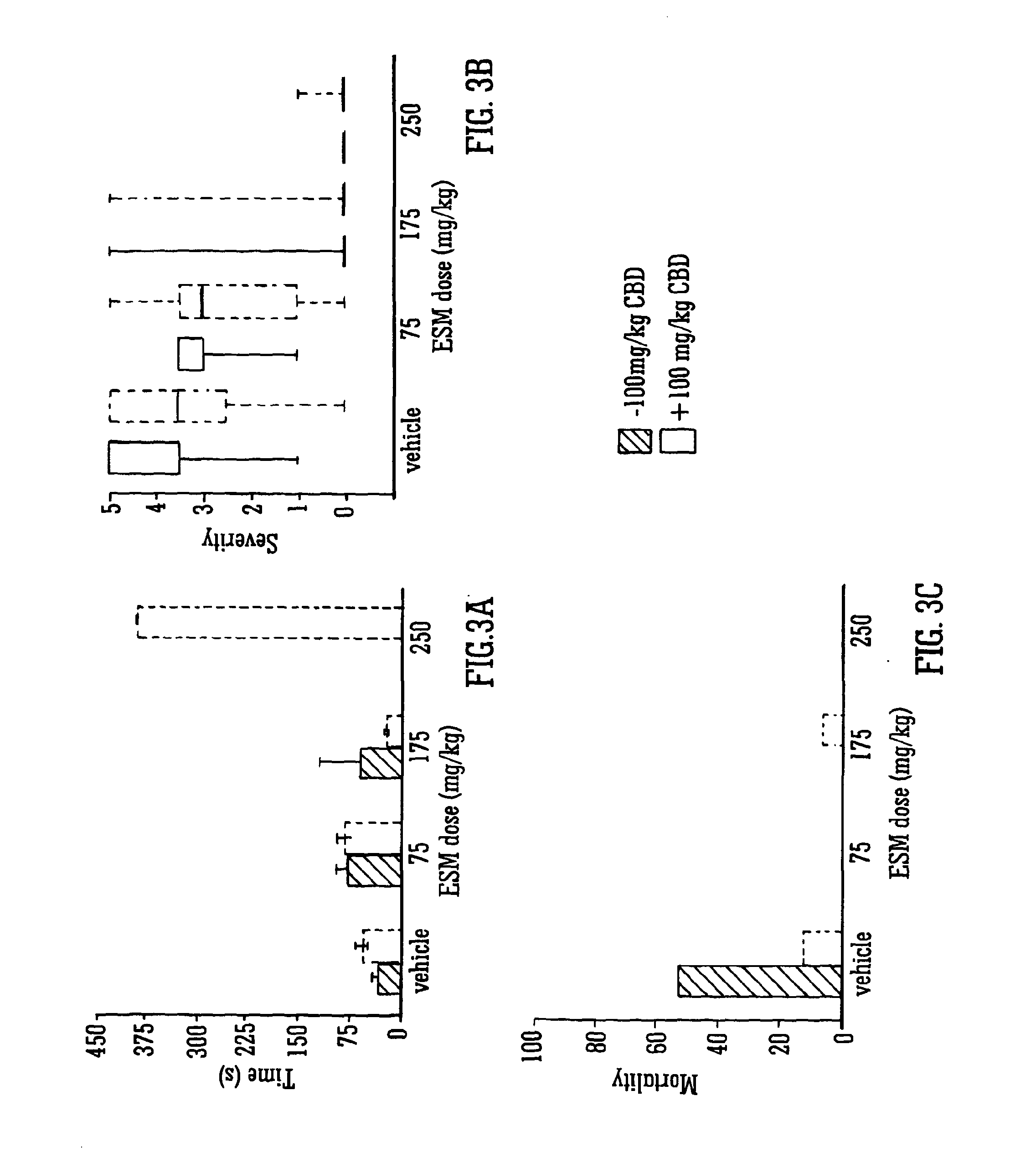
[0088]The Use of the Phytocannabinoid CBD in Combination with a Standard Anti-Epileptic Drug (SAED) in the Pilocarpine Model of (Temporal Lobe) Epilepsy
Methodology:
[0089]Isolated CBD was injected intra-peritoneally (IP) in the standard vehicle (1:1:18 ethanol:Cremophor:0.9% w / v NaCl) at doses of 50, 100 and 200 mg / kg alongside animals that received vehicle alone at a matched volume. 15 minutes later methylscopolamine (1 mg / kg; to reduce peripheral muscarinic effects of pilocarpine) was administered followed, 45 minutes later by pilocarpine (380 mg / kg, IP) administration.
Results:
[0090]FIG. 4 demonstrates the anti-convulsant effects of a combination of CBD and valproate in the pilocarpine model of epilepsy. These data show that the combination of the CBD and valproate was able to increase the latency of onset of the seizure.
[0091]It can be seen from the data illustrated in FIG. 5 that in addition to increasing the latency of onset of the seizure the combination of CBD and valproate wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass flow rate | aaaaa | aaaaa |
| Refractory | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com