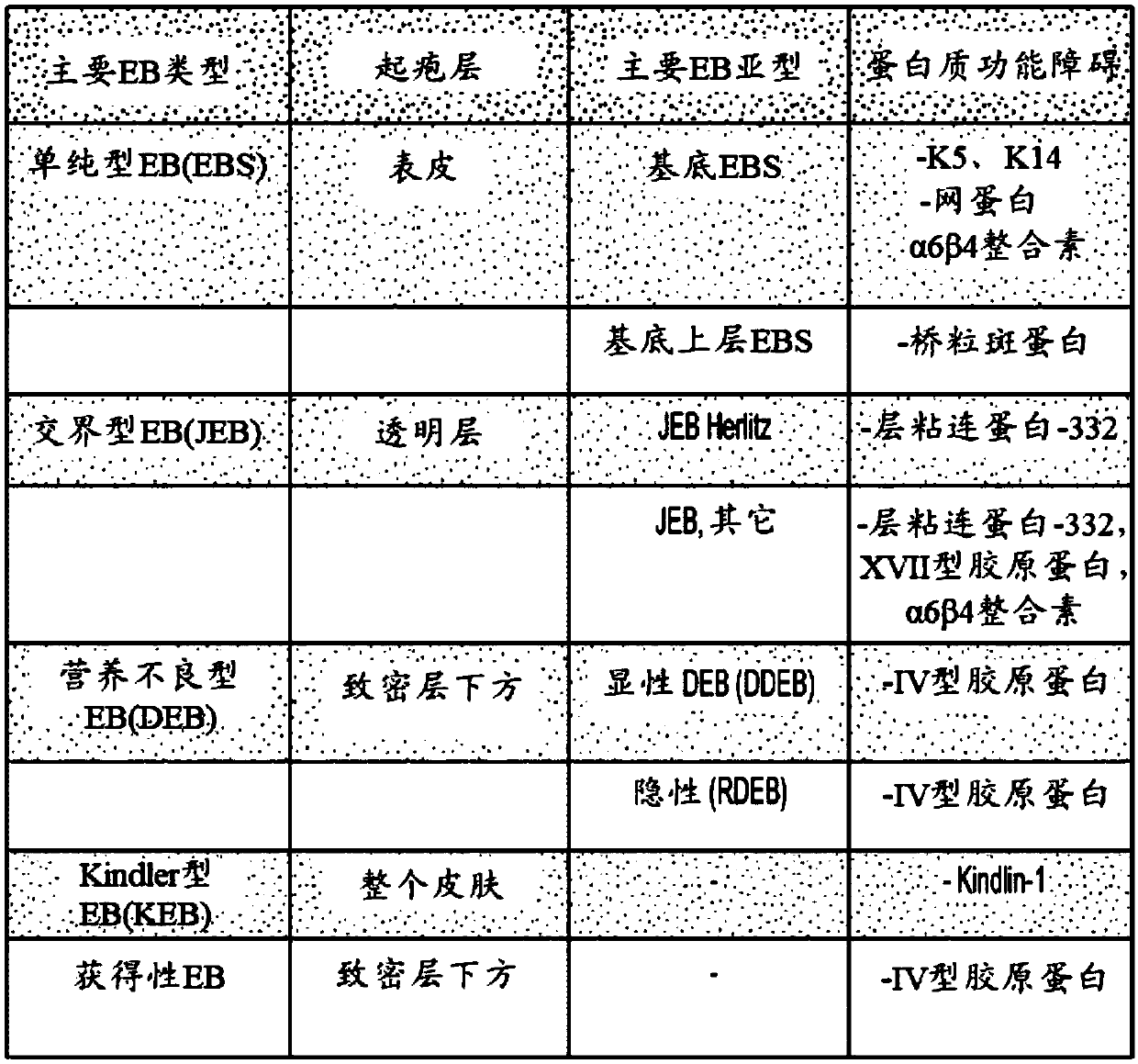
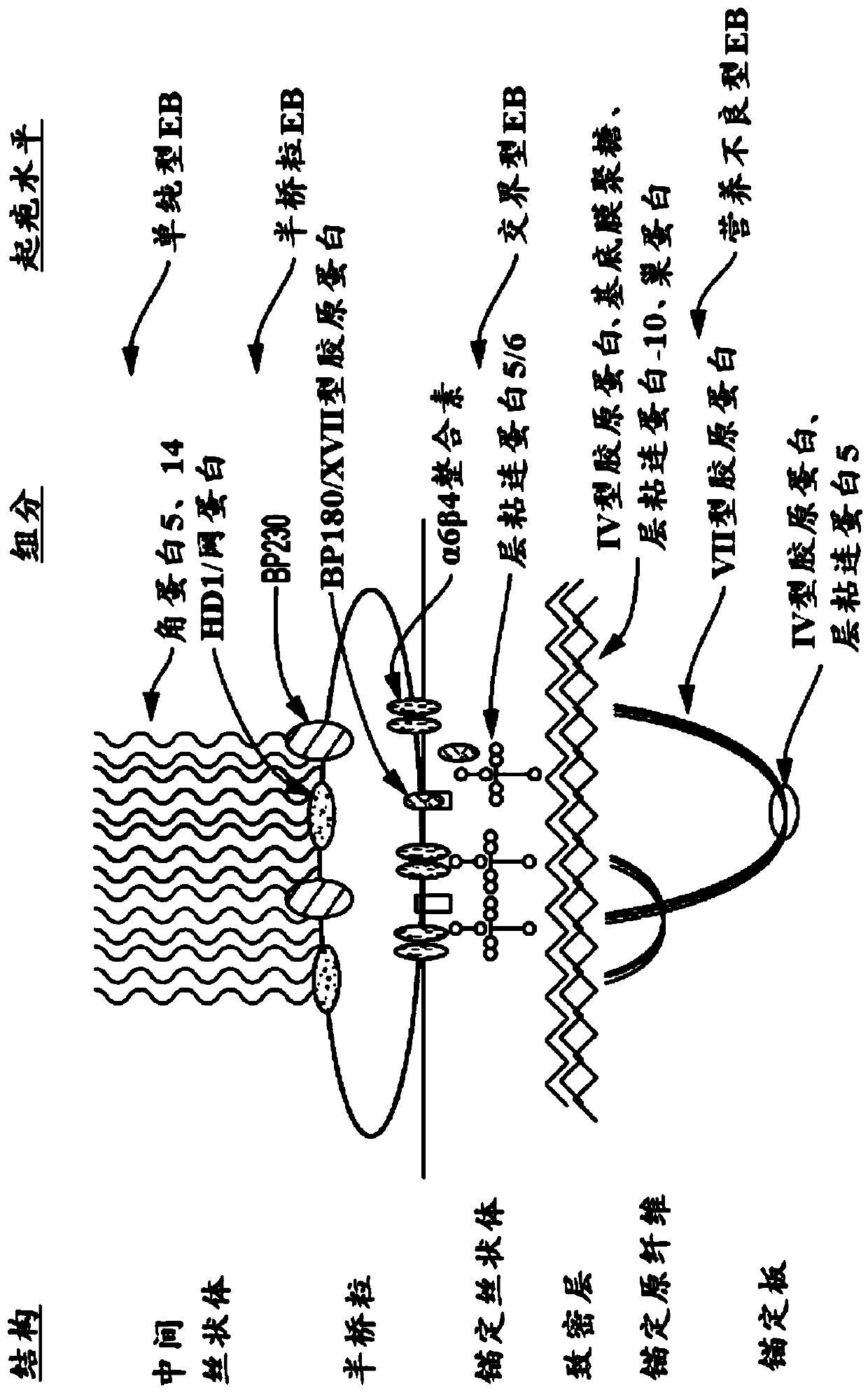
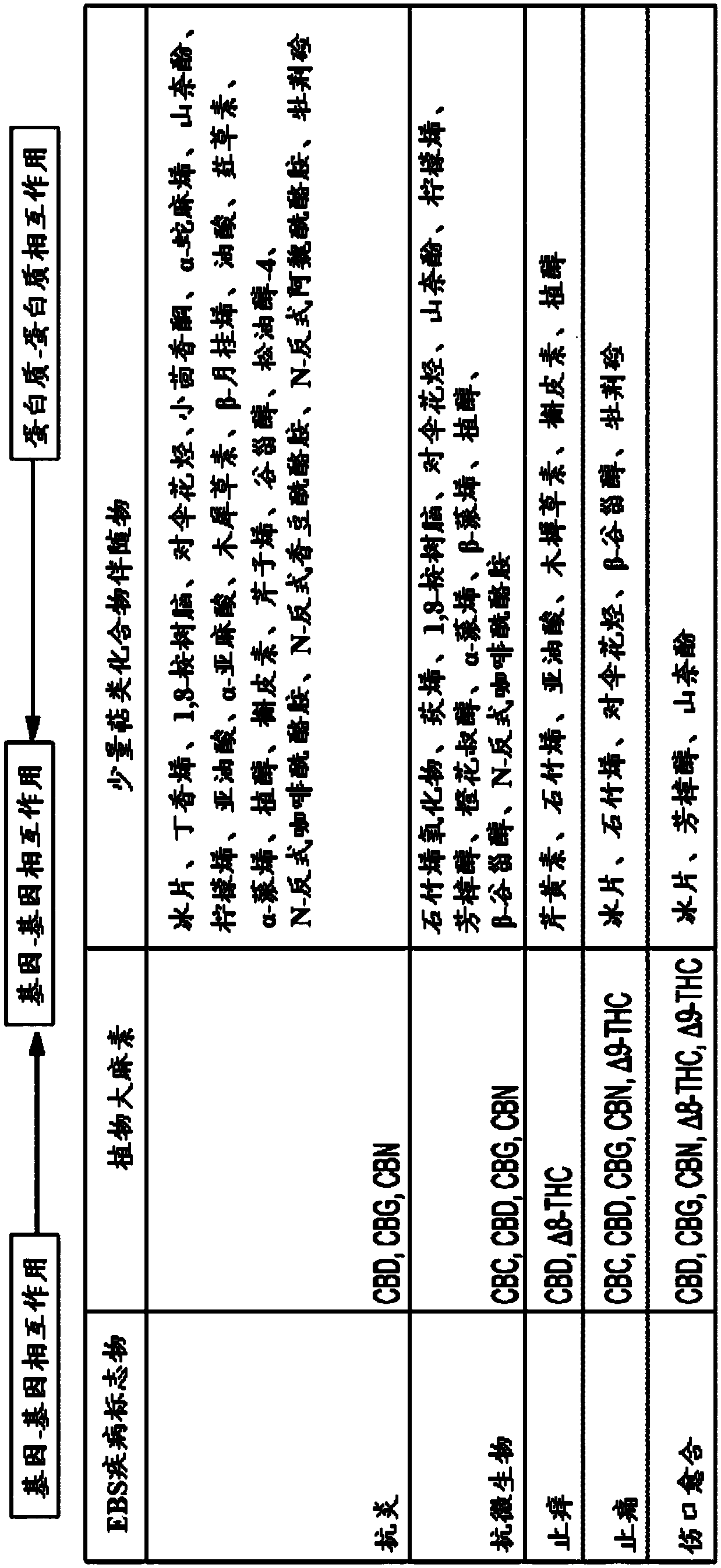
Use of topical formulations of cannabinoids in the treatment of epidermolysis bullosa and related connective tissue disorders
A Cannabinoid, Topically Applied Technology, Applied to Cannabidiol and Cannabidiol: Cannabis, Topically Applied Cannabinoids, Improved Methods and Compositions for Topical Treatment of Such Diseases and Conditions, Epidermolysis Bullosa Simplex Field , can solve the problems of increasing the risk of serious infection in patients and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0292] Example 1: Cannabinoids regulate keratin expression in HaCat cells
[0293] The materials to be tested are the phytocannabinoid cannabidiol (CBD), cannabidiol acid (CBDA), cannabidiol (CBN), cannabidiol: cannabidiol (1: 0.1 μM), cannabidiol: cannabidiol (0.1 : 1μm) and cannabidiol: cannabidiol (1:1μM).
[0294] Cell culture was carried out as follows: in Duchenne supplemented with 10(V / V)% fetal bovine serum (FBS; Life Technologies Hungary Ltd.) and an antibiotic mixture (1:100 penicillin and streptomycin; PAA Laboratories GmbH, Pasching, Austria) Modified Eagle Medium (DMEM; Life Technologies Hungary Ltd.) and HaCaT (human immortalized keratinocyte cell line) was cultured in Antimicotic (1:200; Life Technologies Hungary Ltd.).
[0295] Containing 5% CO 2 The cells were cultured at 37°C in a humidified atmosphere, the medium was changed every other day, and the cells were subcultured at 70-80% confluence in all cases. For medication, the medium is changed daily.
[0296] Qu...
Embodiment 2
[0330] Example 2: Topical cannabinoid formulation and skin penetration
[0331] The skin penetration of the formulation described herein is measured according to the following procedure: The formulation is Labrasol, Plo-gel (Poloxomer 407, lecithin, isopropyl palmitate). The formulation is applied to the center part of the circle and wiped onto the skin with a scalpel. Mount the sample on the top of the Franz diffusion cell with the outer layer of the skin facing up. The receptor medium of the Franz cell is filled with phosphate buffer. Install the diffusion cell cover and clamp it. Place the construct in an incubator / shaker at 32°C for 18 hours ( Figure 14 ). Figure 15 show Figure 14 The results of the penetration experiment (◆, 6 hours; ■, 9 hours; ▲, 12 hours).
[0332] Figure 16 Shows the use of Levenberg-Marquardt fitting and based on Figure 15 The result is a mathematical model of the cannabinoid skin spread. Figure 17 The area under the curve for allantoin (left pa...
Embodiment 3
[0333] Example 3: Cannabinoids and wound healing
[0334] Figure 19 It is an illustration of a model developed based on the experimental results described in this article. This model illustrates that endocannabinoids and cannabinoid receptors are involved in multiple regulatory systems in the skin.
[0335] Figure 20 It is a graph showing the effect of different mixtures of INM-505 (cannabidiol) and INM-517 (cannabidiol) on type II (K5, K6, and K14) and type I (K15, K16, K17) keratin expression. INM-505 and INM-517 alone or in combination (INM-505:INM-517) generally increase K5, 14, 15, 16, and 17 protein expression in differentiated human keratinocytes (concentration-dependent effect).
[0336] Figure 21 Shows that cannabinoids up-regulate the activity of extra domain A (EDA)-fibronectin in wound healing (left panel). Cannabinoids rescued the inhibitory effect of TGF-β-induced E-cadherin (right panel).
[0337] Transforming growth factor (TGFβ1)β is the main regulator of normal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com