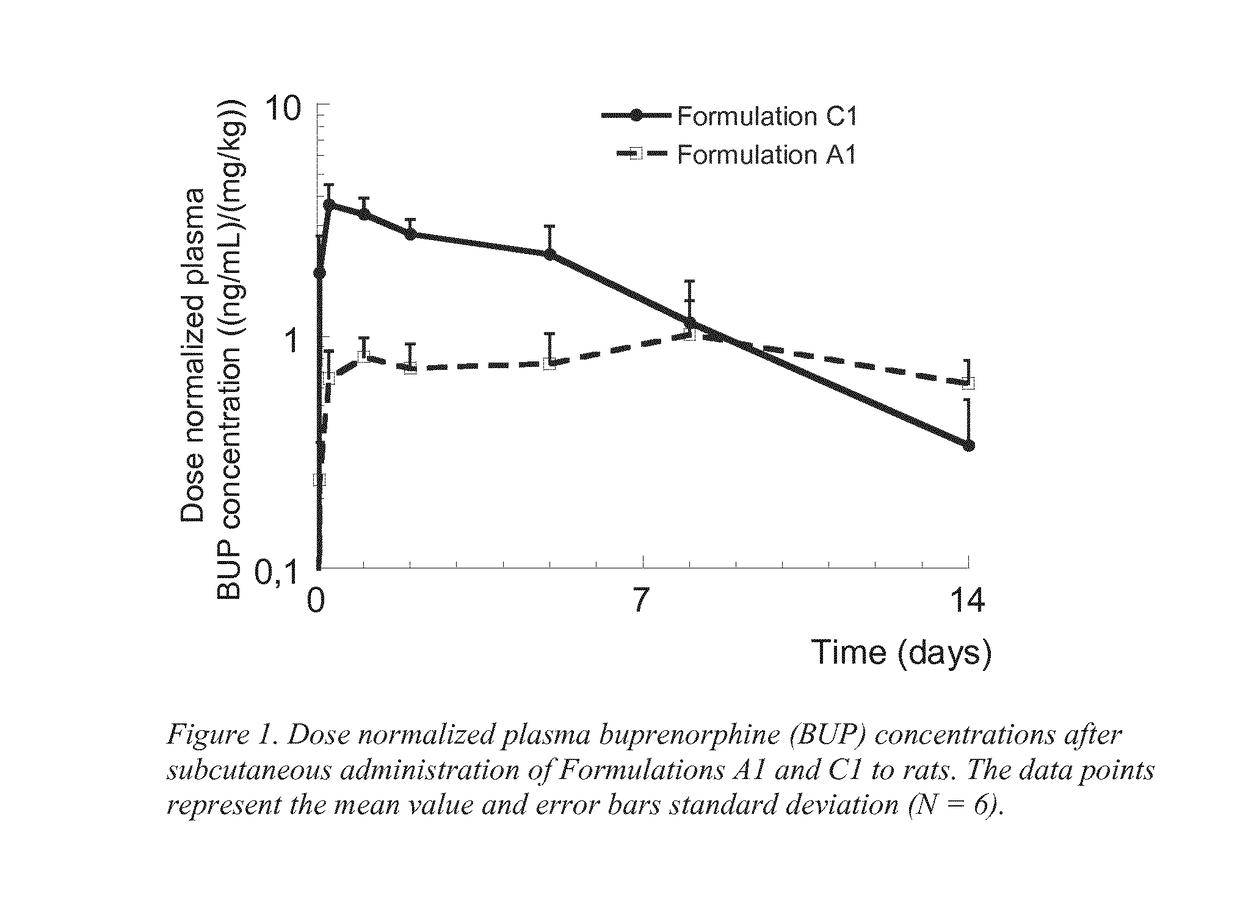
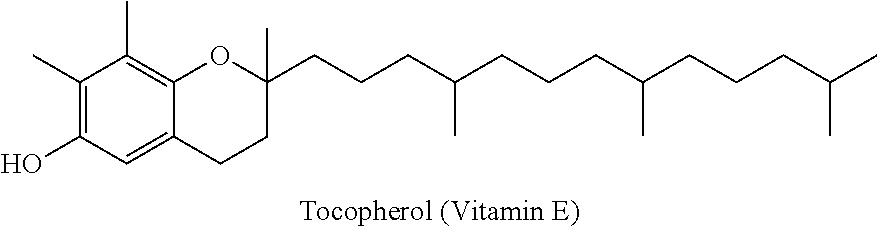
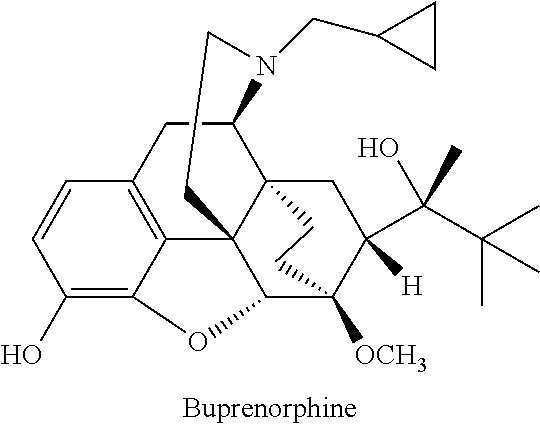
Injectable buprenorphine formulation
a buprenorphine and injection technology, applied in the direction of nervous disorder, non-active ingredients of oil/fat/waxes, drug compositions, etc., can solve the problems of debilitating and unpleasant withdrawal, affecting the release of buprenorphine, and causing serious side effects. achieve the effect of favorable release profiles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Composition Comprising Buprenorphine Base, Sesame Oil and N-Methyl Pyrrolidone (NMP)
[0094]The composition according to Table 1 was prepared by weighing 2.03 g buprenorphine base, 1.80 g NMP and 2.17 g of Sesame oil in a 10 mL injection glass vial. The vial was closed with Flurotec®-coated rubber stopper and aluminum crimp cap followed by end-over-end rotation mixing at ambient room temperature until a liquid, transparent and homogenous formulation was obtained. The formulation was finally subjected to filtration through a sterile 0.22 μm Millex®-GV membrane (Millipore) under nitrogen pressure.
TABLE 1Composition of buprenorphine, triglyceride and solvent (wt %)FormulationBuprenorphineSesame oilNMPA133.836.230.0NMP = N-methyl pyrrolidone
example 2
Compositions Comprising Buprenorphine Base, Triglycerides and Solvents
[0095]The compositions according to Table 2 are prepared by weighing the required amounts of buprenorphine base, triglyceride and solvent in 4 mL injection glass vials. The vials are closed with Flurotec®-coated rubber stoppers and aluminum crimp caps followed by end-over-end rotation mixing at ambient room temperature.
TABLE 2Composition of buprenorphine, triglyceride and solvent (wt %)SesameCastorFormulationBuprenorphineoiloilMCTNMPDMSOB133.8—36.2——30.0B225.4—44.6——30.0B316.9—63.1——20.0B440.0—20.0——40.0B525.444.6——30.0—B616.963.1——20.0—B740.020.0——40.0—B833.8——36.230.0—B925.4——44.630.0—B1016.9——63.120.0—B1140.0——20.040.0—DMSO = Dimethyl sulphoxideNMP = N-methyl pyrrolidoneMCT = Medium Chain Triglycerides (e.g. Labrafac Lipophile WL 1349, Gattefossé, France)
example 3
Composition Comprising Low Drug Load Buprenorphine Base, Triglyceride and Solvent
[0096]The composition according to Table 3 was prepared by weighing 0.0424 g buprenorphine base, 0.400 g Ethanol and 3.558 g of Castor oil in a 10 mL injection glass vial. The vial was closed with Flurotec®-coated rubber stopper and aluminum crimp cap followed by end-over-end rotation mixing at ambient room temperature until a liquid, transparent and homogenous formulation was obtained. The formulation was finally subjected to filtration through a sterile 0.22 μm Millex-GP membrane (Millipore) under nitrogen pressure.
TABLE 3Composition of buprenorphine, triglyceride and solvent (wt %)FormulationBuprenorphineCastor oilEtOHC11.0688.9410.00EtOH = Ethanol
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com