Methods and compositions for modulating opioid withdrawal symptoms
A technology for withdrawal symptoms and compositions, applied in the field of screening compounds and compositions, can solve problems that plague chronic opiate users, ineffective clinical strategies, debilitating withdrawal syndrome, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0104] The following methods and materials were used in the Examples disclosed in this application.
[0105] animal:
[0106] Adult male rats and mice (7-8 week old rats; 6-10 week old mice) were used. Mice and rats were housed on a 12 hr / 12 hr light / dark cycle with ad libitum access to food and water. All experiments were approved by the University of Calgary and Laval University Animal Care Committees and in accordance with the guidelines of the Canadian Humane Society.
[0107] Morphine Dose Paradigm and Nociceptive Behavior Model:
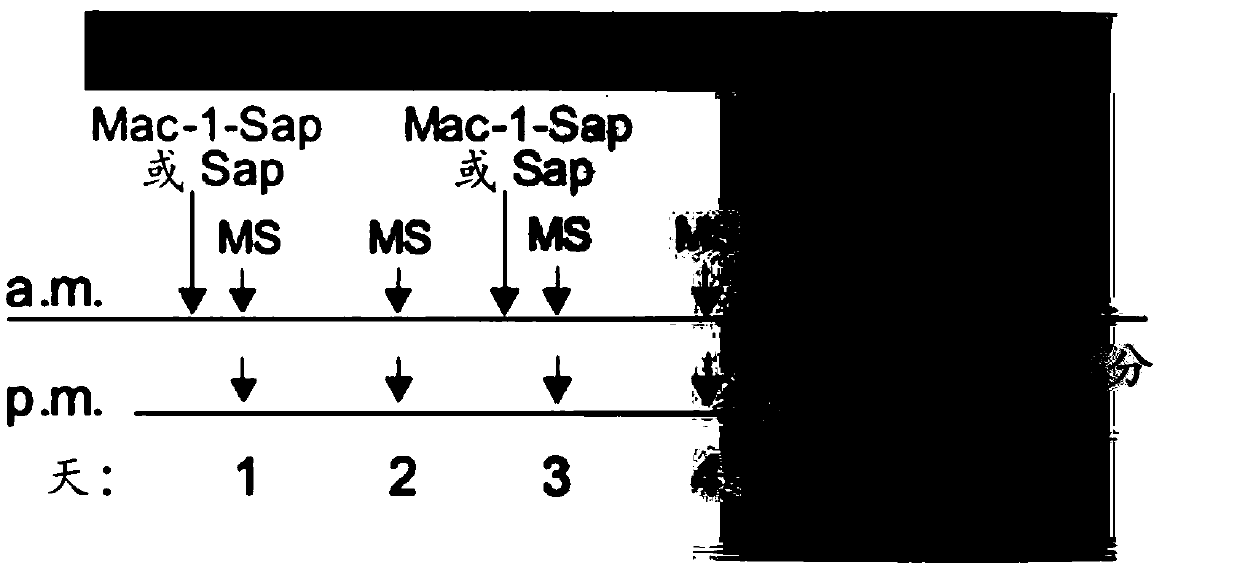
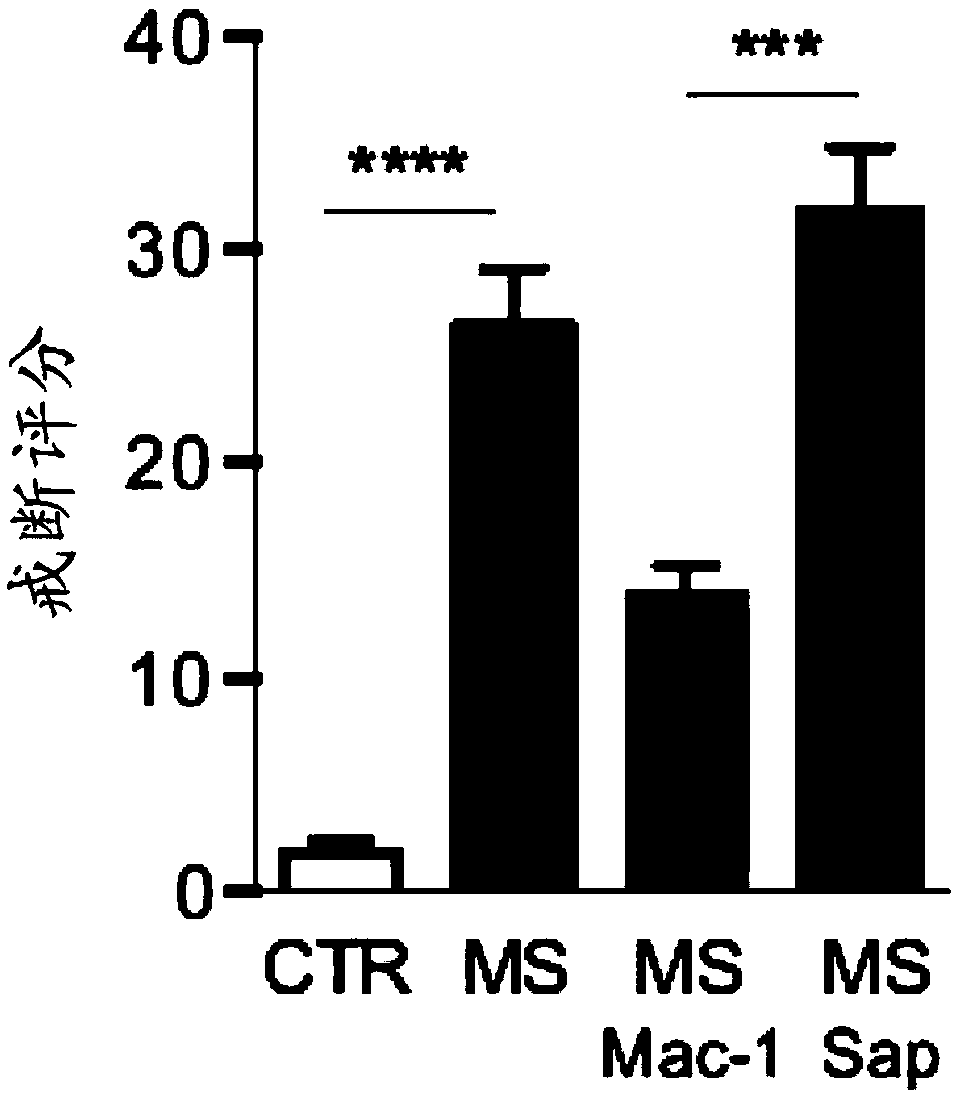
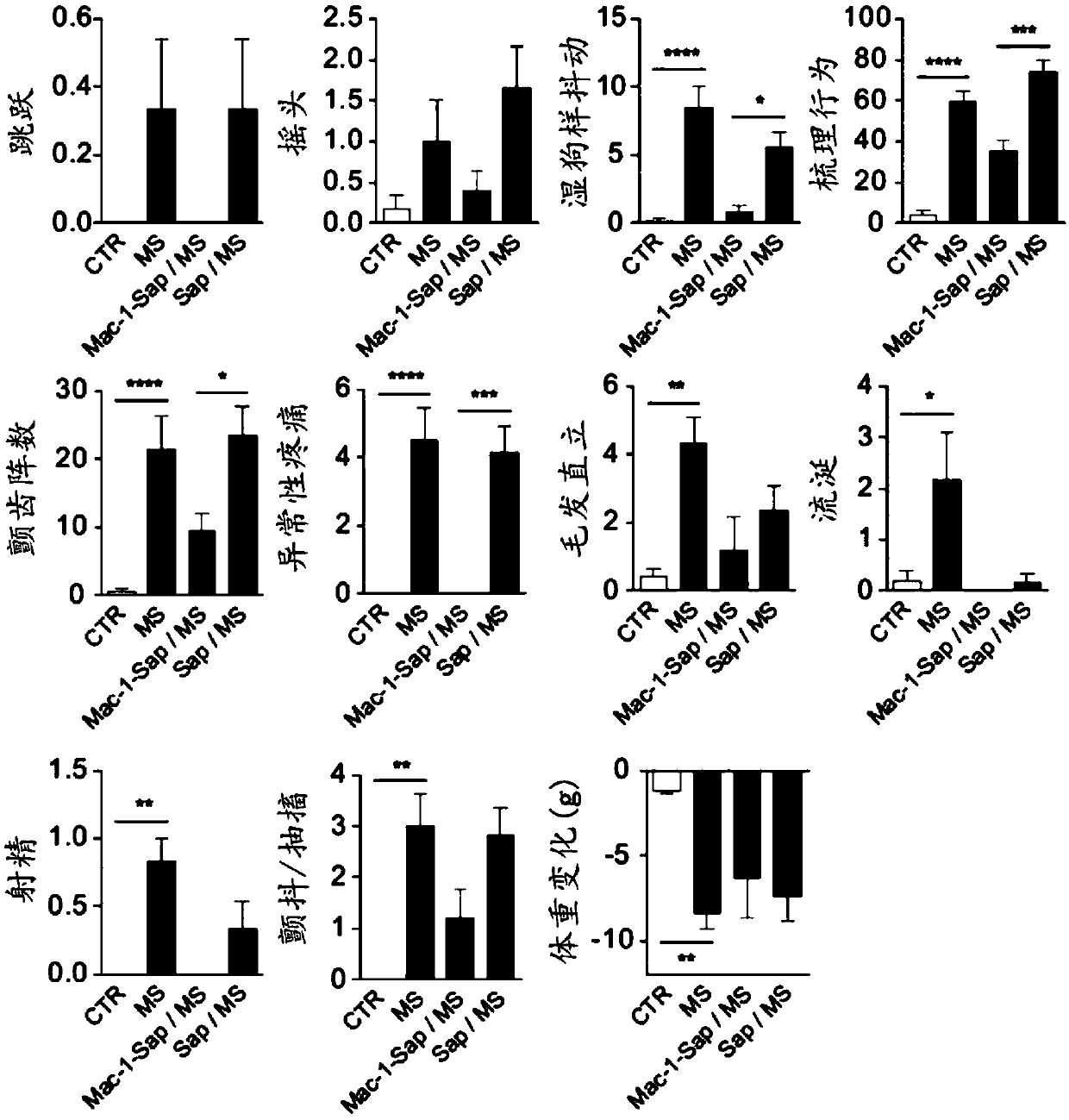
[0108] Morphine sulfate (PCCA) prepared in 0.9% sterile saline solution was injected intraperitoneally twice daily (8 am and 5 pm) into Sprague-Dawley rats (increasing doses from 10 to 45 mg / kg), Cx3cr1-cre::Pnx1 flx / flx , or Pnx1 flx / flx Mice (increasing doses from 7.5 to 50 mg / kg). Thermal nociceptive thresholds were assessed using the tail flick test (rats) and the tail immersion test (mice). For rats, an infrared heat stimulus (Ugo B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com