Deuterated mitragynine analogs as safer opioid modulators in the mitragynine class
A technology of pharmacy, composition, applied in the field of deuterated mitragynine analogs as safer opioid modulators in mitragynines, capable of solving problems such as loss, negative medicine and social cognition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
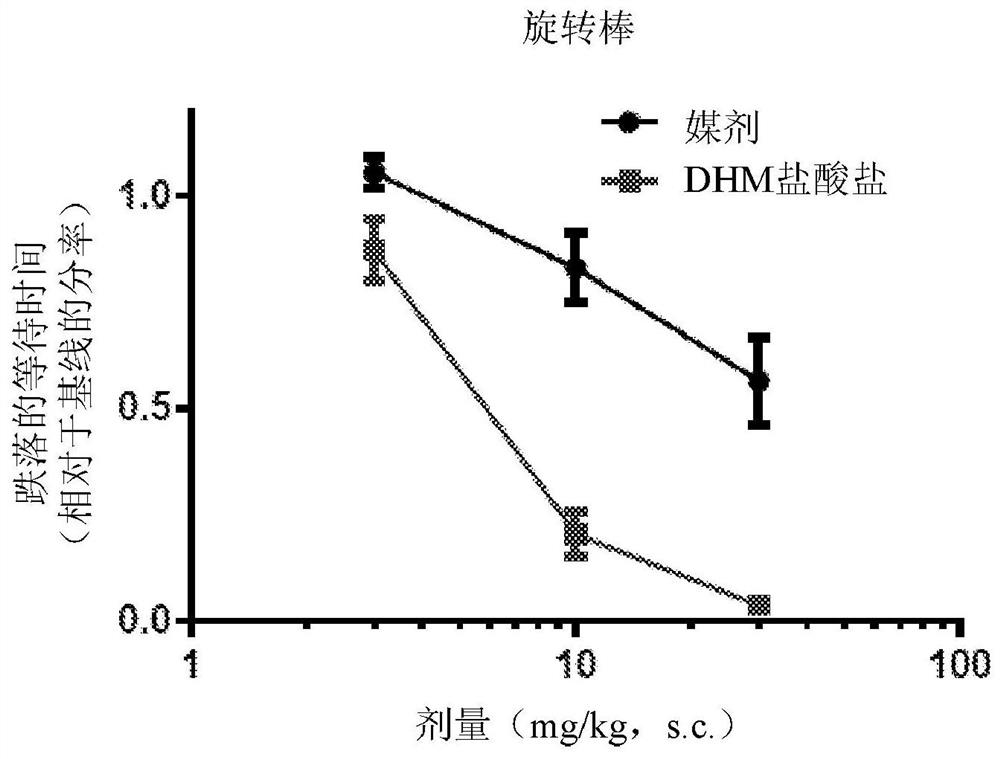
[0654] Example 1: Effect of 3-dehydromitragynine in the rotarod test in mice
[0655] The rotarod test is useful for measuring motor coordination in rodents, and thus for identifying test drugs that induce ataxic effects. 3-Dehydromitragynine (DHM) reduced the performance of mice in this test in a dose-dependent manner, suggesting that this compound impairs motor coordination ( figure 1 ).
[0656] Animals: 7-week-old male C57BL / 6J mice (n=10 / treatment) purchased from The Jackson Laboratory (Bar Harbor, ME) were used for this study. Animals were housed in groups of five and acclimatized for 1 week prior to testing. Mice had access to food and water ad libitum and were maintained on a 12 hour light / dark cycle. All tests were performed in a light cycle.
[0657] Drug: DHM hydrochloride dissolved in double distilled H containing 10% N-methyl-2-pyrrolidone (NMP) 2 O middle. Drug or vehicle was administered subcutaneously in a volume of 10 mL / kg body weight 15 minutes befor...
example 2
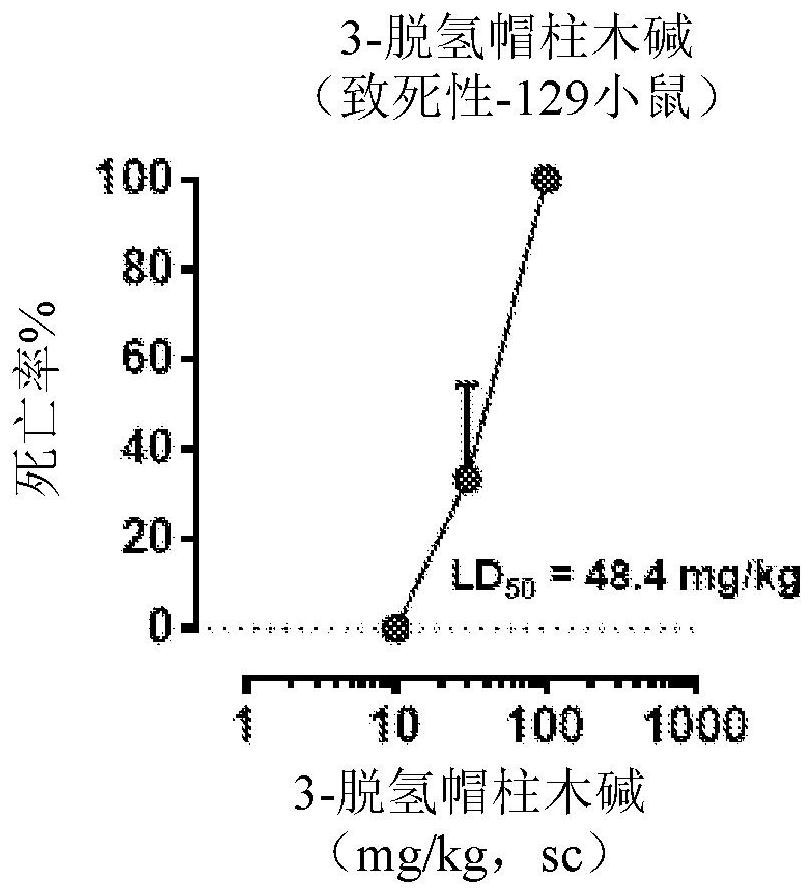
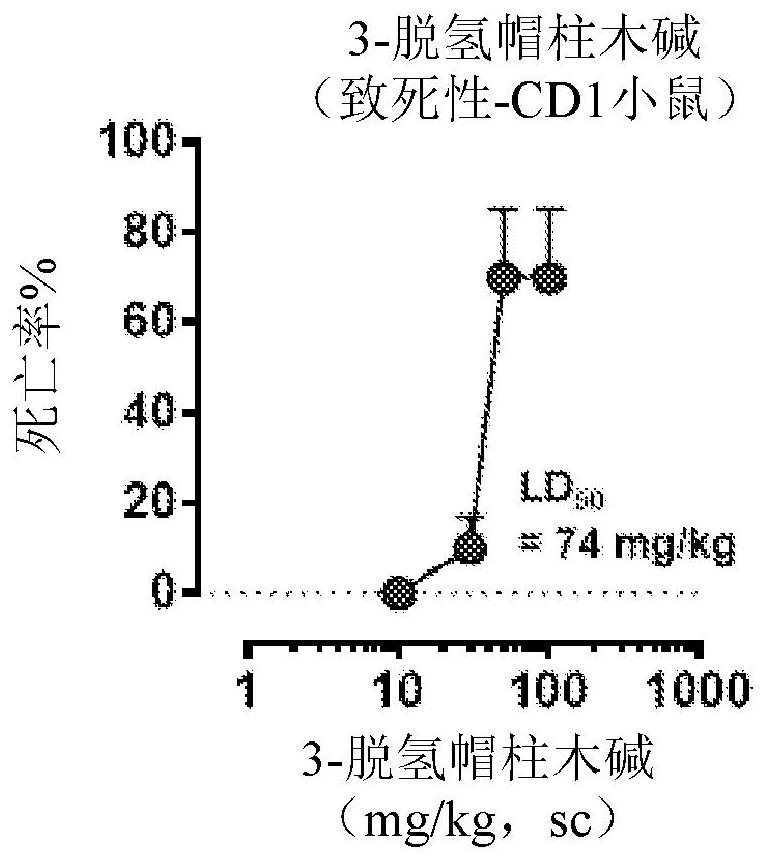
[0659] Example 2: Lethality of 3-dehydromitragynine in mice
[0660] Treatment of mice with 3-dehydromitragynine (DHM) resulted in death in a dose-dependent manner in two different strains, demonstrating the general toxicity of this metabolite (Figure 2).
[0661] Lethality assay: Groups of mice (n=6 per dose, 129Sv6 or CD-1 strain) were treated subcutaneously (s.c.) with different doses of DHM, and lethality was tested 24 hours after drug administration.
example 3
[0662] Example 3: Attenuated Formation of 3-Dehydromitragynine in Liver Microsomes
[0663] In human liver microsomes (HLM), both 7-hydroxymitragynine (7-OH) and 3-dehydromitragynine (DHM) are formed as metabolites of mitragynine (Figure 3) . For example, the deuteration of the 3-position of mitragynine in 3-deutero-mitragynine (3-DM) weakens the formation of DHM by kinetic isotope effect ( Figure 3A ), without affecting the oxidative metabolism at position 7 to give 3-deutero-7-hydroxymitragynine (3-d-7-OH, similar to 7-OH formed from mitragynine) ( Figure 3B ). Thus, 3-DM offers a significant advantage over mitragynine in that it attenuates the formation of the toxic metabolite DHM while having no effect on the formation of the active metabolite 3-d-7-OH.
[0664] HLM metabolite formation: Pooled HLMs from 50 adult male and female donors (XenoTech H0630, lot 1610016) were used. Microsomal incubations with mitragynine and 3-DM were performed in 5 aliquots of 40 μL eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com