Use of an opioid receptor antagonist for the treatment or prevention of gastrointestinal tract disorders
A technology for the use of opioid substances, which is applied in the field of use of opioid receptor antagonists for gastrointestinal diseases, and can solve problems such as aggravating postoperative intestinal obstruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Pharmacokinetics and pharmacology of compound I in non-clinical models
[0074] Efficacy of Compound I in the CNS compared to naltrexone
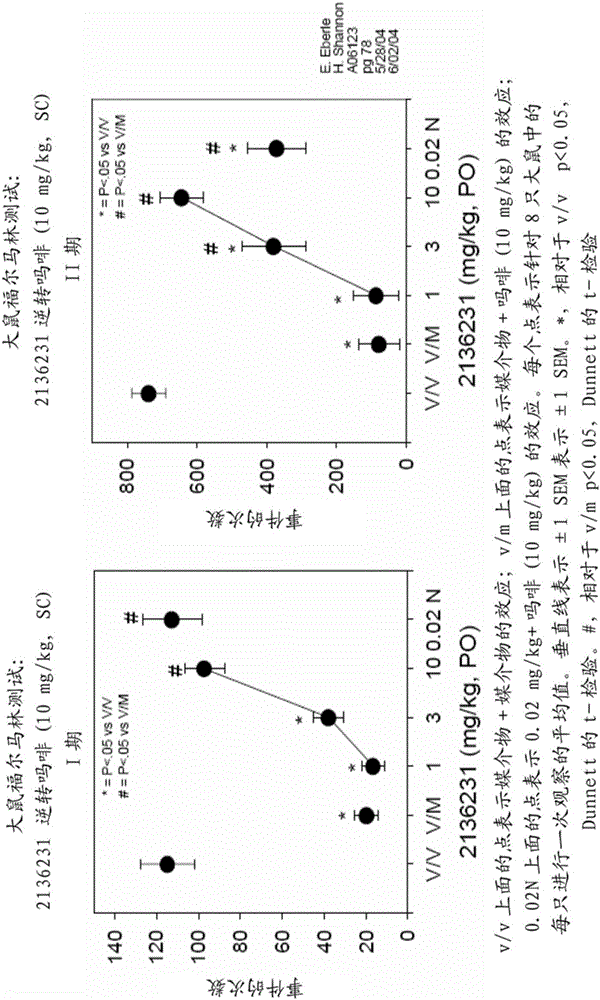
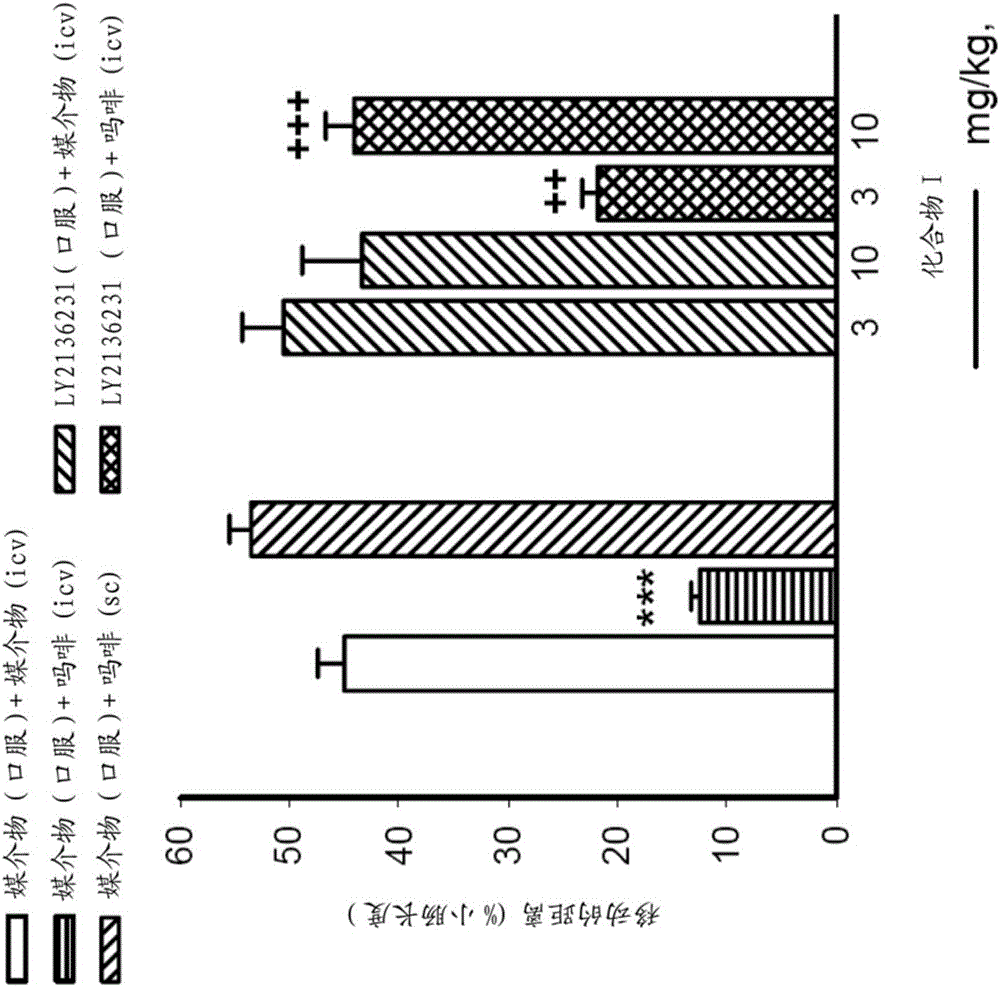
[0075] Rats were injected subcutaneously with stimulating formalin and with 10 mg / kg morphine. The analgesic effect was determined by the number of times (events) in which rats reduced licking or paying attention to the site of formalin injection. Rats were dosed with 0.02 mg / kg naltrexone (subcutaneous administration) or 1, 3 or 10 mg / kg compound I (oral administration) to determine the compound's ability to reverse the analgesic effect of morphine. Naltrexone was found to be approximately 100-fold more potent than compound I in reversing the effects of morphine in the rat CNS ( figure 1 ). Naltrexone and Compound I have similar affinities for μ-opioid receptors. Different experiments showed that Compound I has high oral bioavailability in rats. The analgesic effects induced by reversal of morphine by opioid receptor...
Embodiment 2
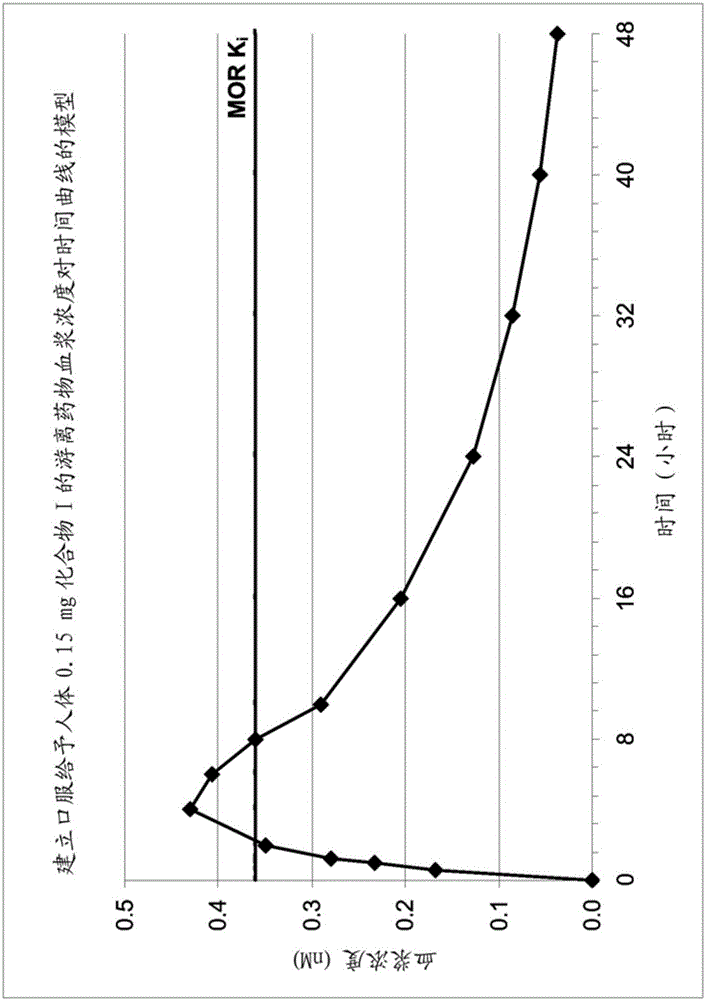
[0080] Example 2: Calculation of an effective dose of Compound I for the treatment of OIC or OBD in humans and determination of a sufficient amount to act peripherally without compromising central analgesia or producing central opioid withdrawal symptoms
[0081] The nonclinical animal studies described above showed that Compound I exhibited some peripheral effects in mice and rats, and further described the main mechanism by which Compound I was excreted from the CNS (i.e., the transporter P- gp substrate). However, the key question is whether these data are relevant to humans and what impact they have on the therapeutic utility of Compound I for the treatment of disorders in humans.
[0082] The main question is whether Compound I can act sufficiently peripherally in humans to treat conditions, particularly OIC or OBD, which depends on its selectivity for peripheral opioid receptors versus antagonism of opioid receptors in the CNS Antagonism may otherwise impair centrally m...
Embodiment 3
[0099] Example 3: Phase I Multiple Escalating Dose Clinical Study with Compound I; Preliminary Results
[0100] The purpose of the phase I multiple escalating dose clinical study was to evaluate the safety, tolerability, pharmacokinetics, and clinical effects of multiple escalating doses of Compound I administered twice daily (BID) due to the Pain Subjects with opioid-induced constipation (OIC) on long-term opioid therapy. The first part of the study was a randomized, double-blind, placebo-controlled, multiple escalating dose study, during which subjects received 4 oral doses of the study drug over 2 days while confined to the study unit. ). Each of the 0.10 mg, 0.25 mg, 0.35 mg, 0.50 mg, and 0.75 mg BID dose groups included 4 subjects, and randomization was unbalanced, with Compound I treatment groups (n=3) versus placebo treatment groups (n=1) The ratio was 3:1. Only 2 subjects were included in the 0.75 mg BID group. Evaluation of clinical effects included the following m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com