Treatment of opioid withdrawal
A technology for opioid drug withdrawal, which is applied in the field of treatment and/or prevention of opioid drug withdrawal, and can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0227] The preparation method of the compound of formula (I) is as described in PCT / AU2016 / 050588.
[0228] Method of administration
[0229] As defined herein, a compound of formula (I) or a pharmaceutically acceptable salt or prodrug thereof may be administered by any suitable means, for example, orally, rectally, nasally, vaginally, topically (including buccal and lingual) ), parenterally, eg, by subcutaneous, intraperitoneal, intravenous, intramuscular, or intracerebral injection, inhalation, insufflation, infusion, or implantation techniques (eg, as sterile injectable aqueous or non-aqueous solutions or suspensions).
[0230] The compounds of the present invention may be provided as pharmaceutical compositions, including oral, rectal, nasal, topical (including buccal and sublingual), parenteral (including intramuscular, intraperitoneal, subcutaneous and intravenous), or in a form suitable for administration by inhalation Or administered in the form of insufflation. Comp...
Embodiment 1
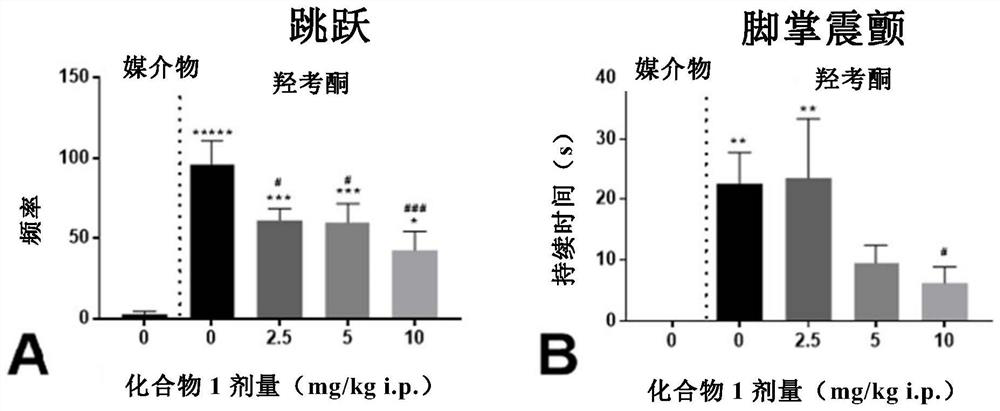
[0285] This example describes experiments in a C57BL / 6 mouse opioid withdrawal model (naloxone-facilitated withdrawal following oxycodone administration) and the potential of compounds of the invention to treat withdrawal symptoms.
[0286]
[0287]
[0288] drug
[0289] All drugs used in this study were dissolved in physiological saline and administered in a volume of 10 ml / kg.
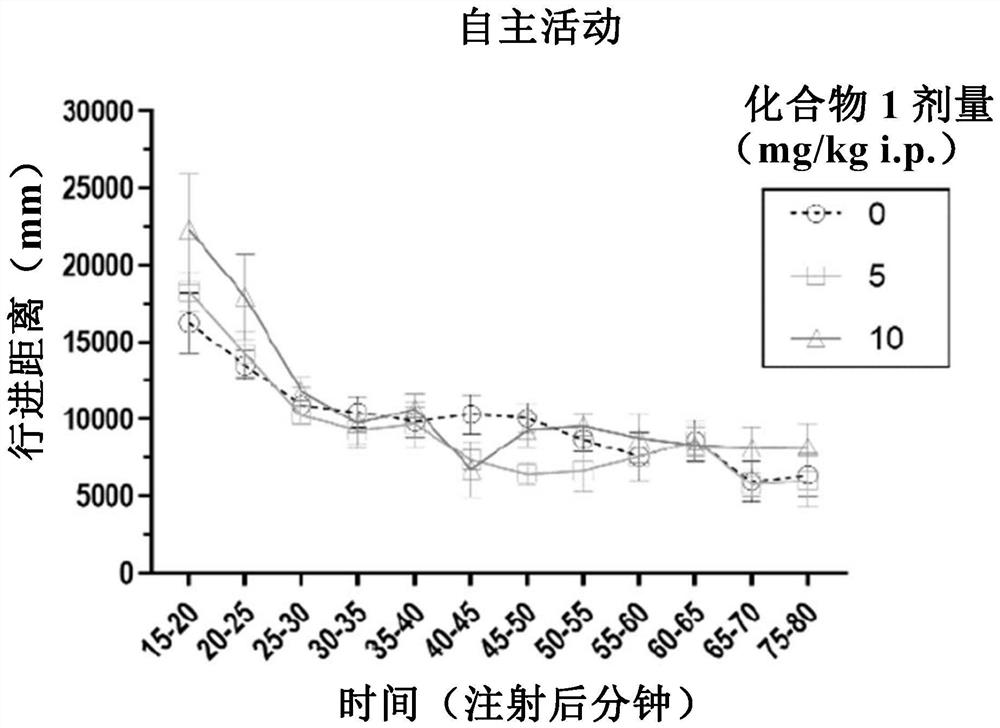
[0290] Experiment 1.1: The effect of CMPD1-2HCL on field exercise ability
[0291] The purpose of this experiment was to demonstrate that CMPD1-2HCL does not cause potentially confounding effects on locomotor activity at doses of 5 and 10 mg / kg. N=18 adult male C57BL / 6 mice received 0, 5 or 10 mg / kg CMPD1-2HCL (i.p.; n=6 per group). Fifteen minutes after receiving intraperitoneal injection of CMPD1-2HCL, the mice were individually placed in a new 40(l)x 40(w)x 40(h)cm locomotor testing field. The testing session was captured by an overhead camera and the video was analyzed using the automat...
Embodiment 2
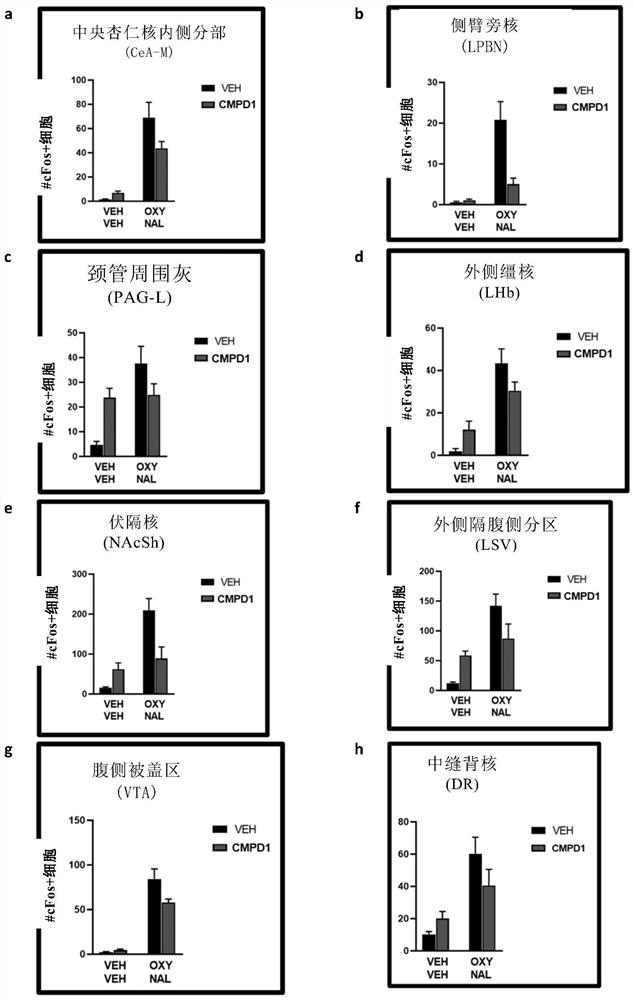
[0327] In this example, the ability of compound 1 to inhibit c-fos protein expression induced by naloxone-induced opioid withdrawal in the brain was investigated. C-fos is a protein marker of neural activation. Compound 1 was administered as the dihydrochloride salt (CMPD1-2HCL).
[0328] method
[0329] N=40 male C57BL / 6 mice were divided into the following four groups:
[0330] (1) VEH, VEH (n = 10)
[0331] (2) VEH, CMPD1-2HCl (n = 10)
[0332] (3) OXY, VEH (n = 10)
[0333] (4) OXY, CMPD1-2HCl (n = 10)
[0334] Mice in the oxycodone (OXY) group received intraperitoneal injections of oxycodone for 9 days with increasing doses of 9, 17.8, 23.7 and 33 mg / kg (twice a day on days 1-8, with dose increases every other day). The morning and afternoon doses are separated by 7 hours. Vehicle group (VEH) mice received injections of vehicle saline instead of oxycodone. One hour and 45 minutes after the morning injection on day 9, mice were intraperitoneally injected with CMPD1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com