Medicinal product and method for treatment of conditions affecting neural stem cells or progenitor cells
a neural stem cell and product technology, applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of neurological and cognitive deficits, damage to the brain, etc., and achieve the effect of modulating the proliferation and/or differentiation of neural stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
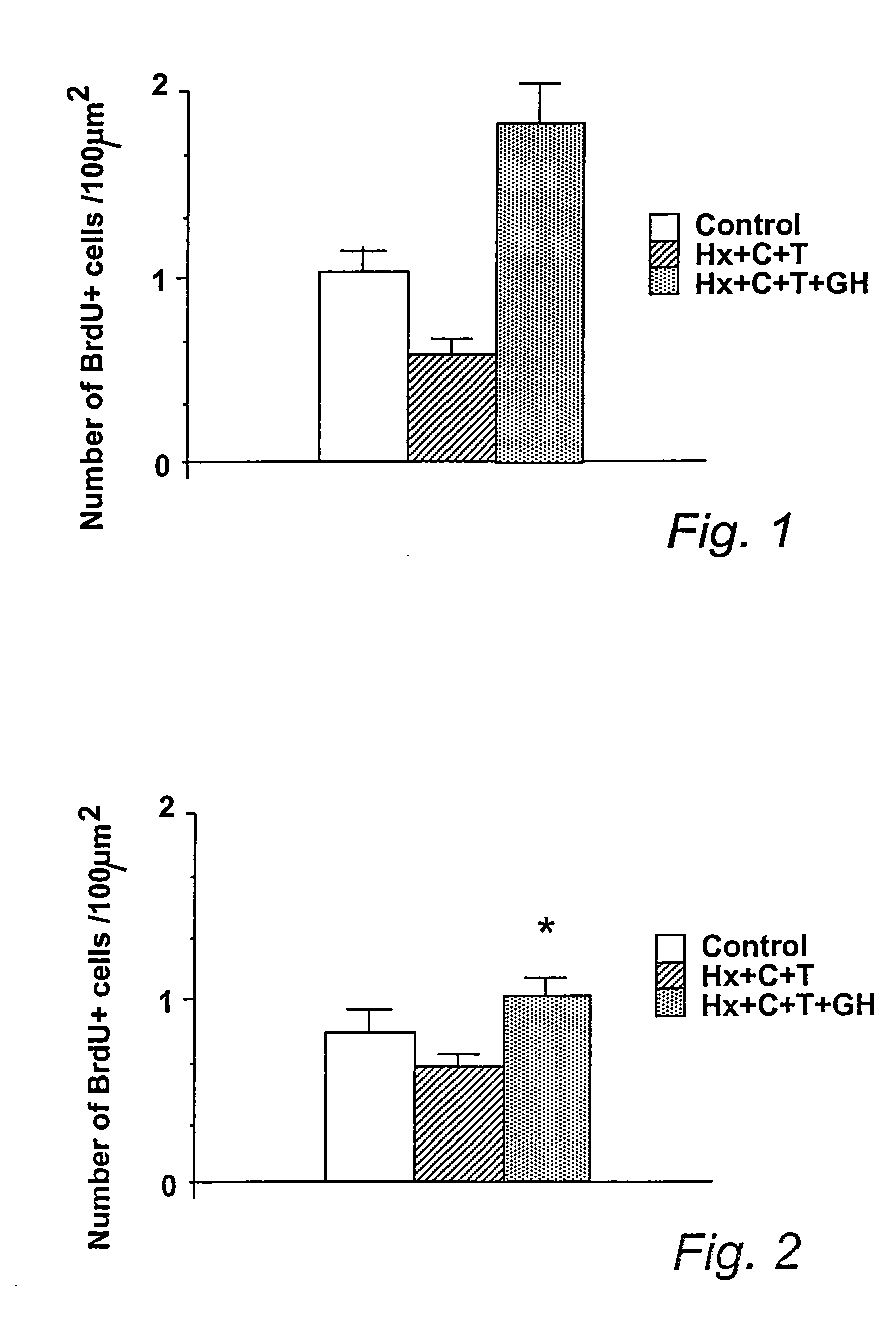
[0039] In this example, the density of BrdU-positive cells in the dentate gyrus of hypophysectomized (Hx) rats treated according to the invention with growth hormone (GH), cortisol (C), and L-thyroxine (T) was compared to the density of BrdU-positive cells in the dentate gyrus of hypophysectomized rats treated with cortisol (C), and L-thyroxine (T), and to the density of the same cells for an untreated unoperated control group.
[0040] Fisher rats (Harlan Sprague Dawley) which were intact or hypophysectomized at 50 days of age were maintained under standardized conditions of temperature (24-26° C.), humidity (50-60%) and with lights on between 0500 and 1900 h.
[0041] The rats had free access to standard laboratory chow and water. Hormonal treatment started 7-10 days after hypophysectomy. All the hypophysectomized rats were given cortisol phosphate (400 μg / kg / day; Solu-Cortef, Upjohn, Puurs, Belgium) and L-thyroxine (10 μg / kg / day; Sigma, USA) diluted in saline as a daily subcutaneous ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com