Particulate drug delivery
a technology of particle and drug delivery, applied in the direction of powder delivery, microcapsules, cyclic peptide ingredients, etc., can solve the problems of complex biodistribution and pharmacokinetic responses in vivo, drug loading and encapsulation efficiency control, and significant toxicities, so as to facilitate particle entry into cells, facilitate particle targeting, and enhance particle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Polylactide-Paclitaxel Nanoconjugate Particles
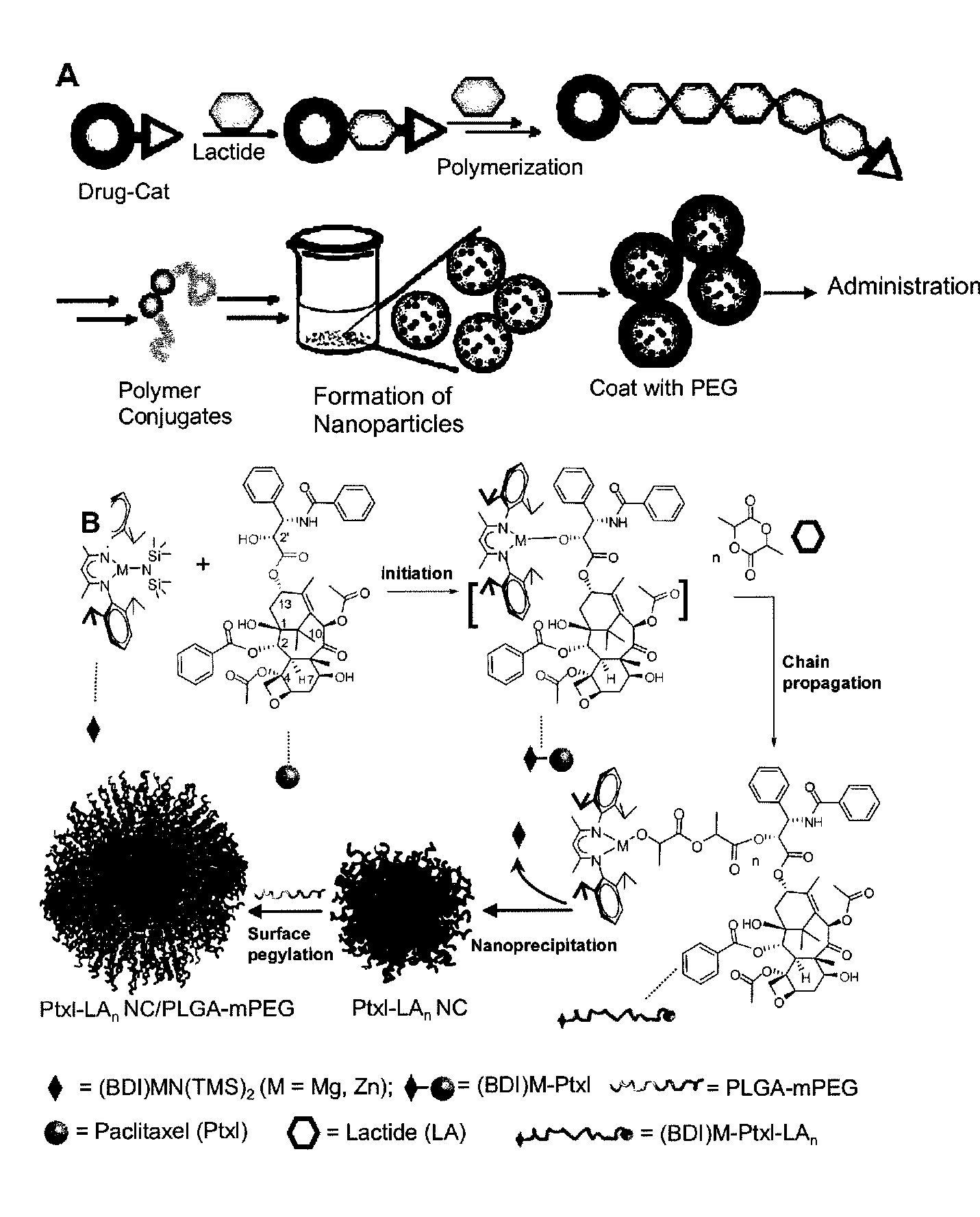
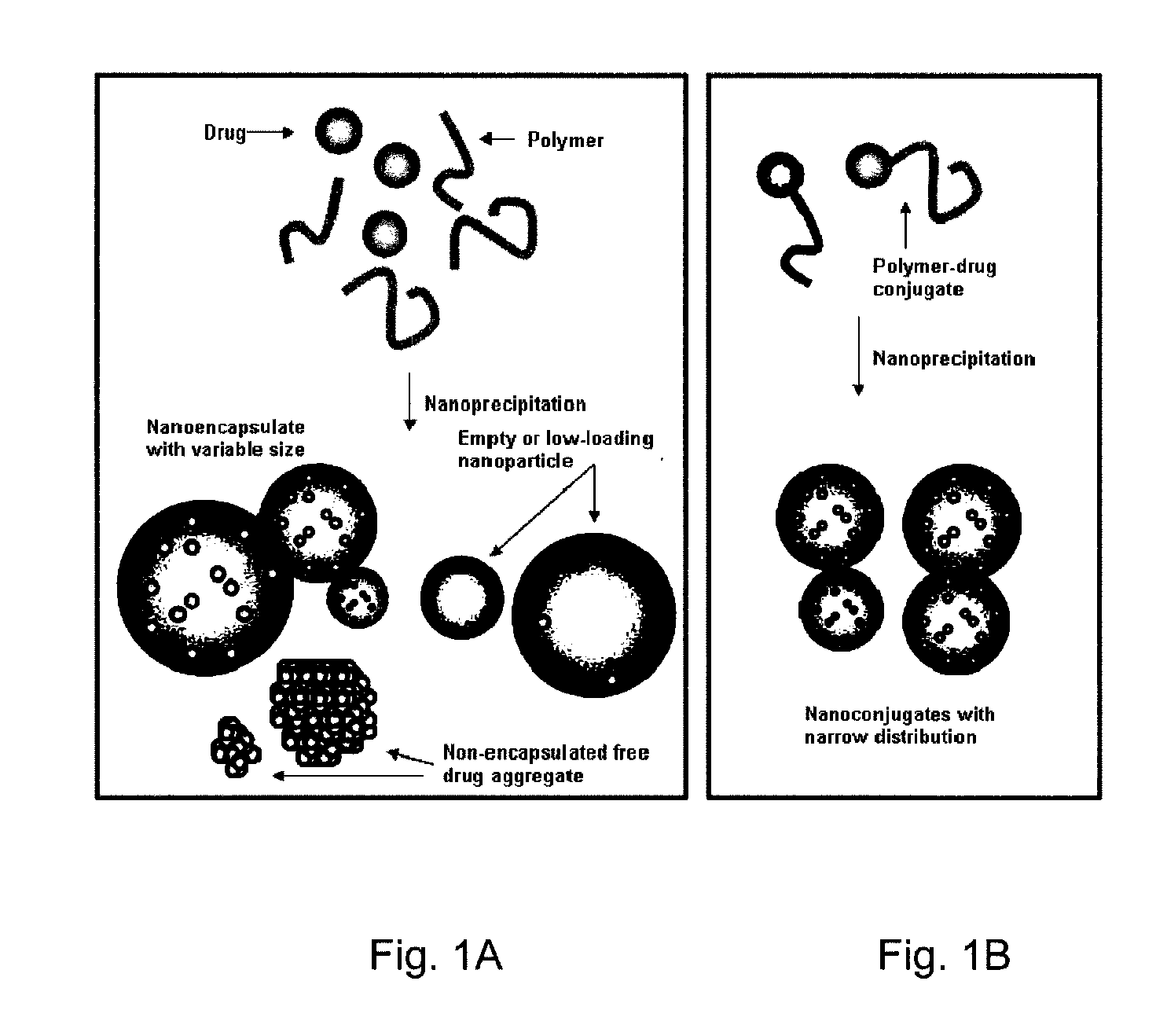
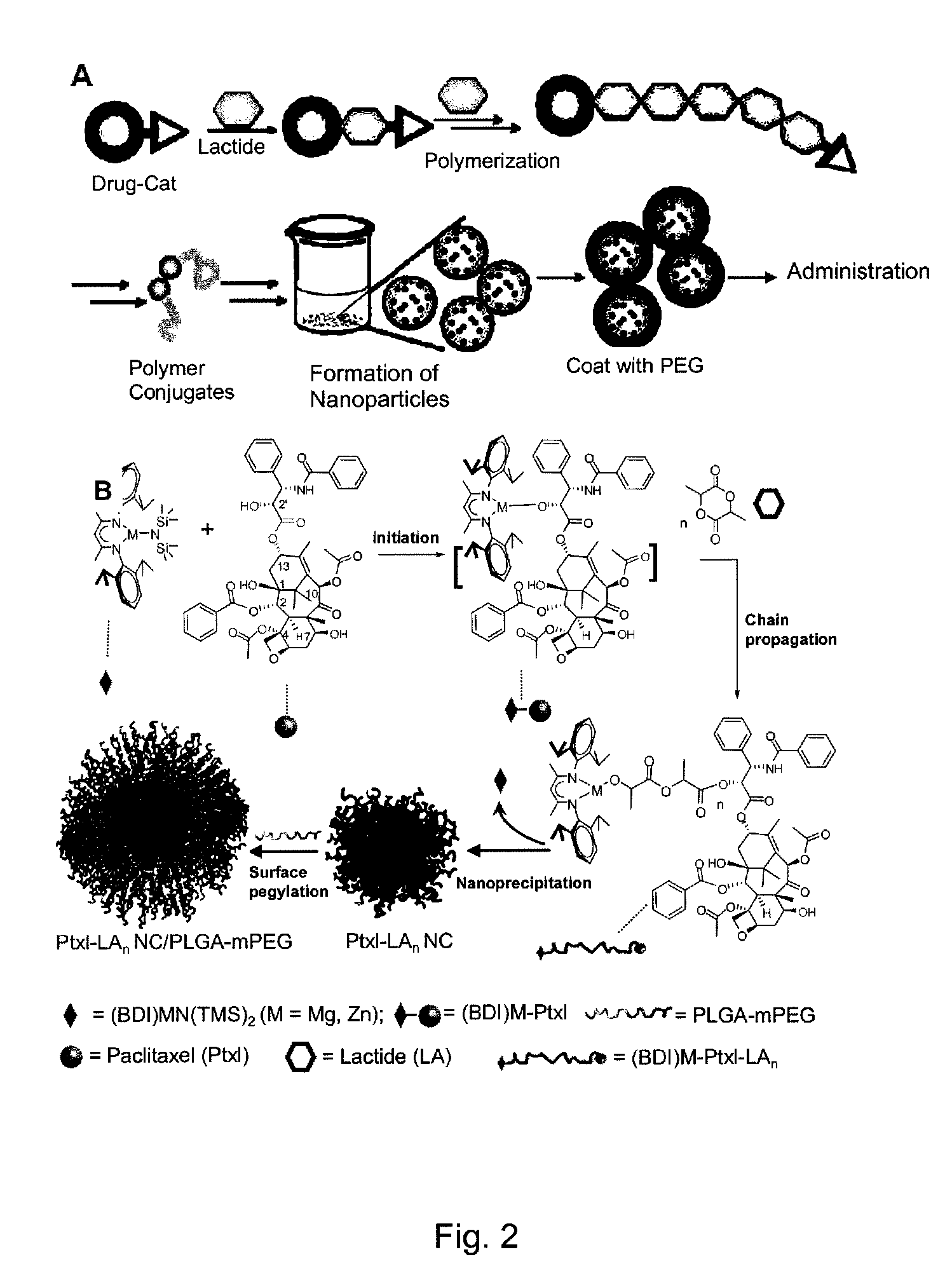
[0111]Nanoparticle design of this invention is based on the use of drugs as initiators in ring-opening polymerization reactions to form drug-polymer (and drug-oligomer) conjugates in which the drug is covalently bonded to the polymer or oligomer. Because the drug is used as the initiator of polymerization, the efficiency of conjugation of the drug to the polymer (oligomer) will be very high, ideally 100%. Additionally, if all of the drug molecules are efficiently incorporated into a living polymerization (e.g. where drug molecules function as initiators), the drug loading percentage can be precisely controlled by adjusting the monomer / initiator ratio.
[0112]To initially demonstrate this strategy, paclitaxel (Ptxl) was used in the presence of an appropriate catalyst to initiate a living polymerization of lactide. Utilization of molecules containing hydroxyl groups as initiators for the ring-opening living polymerization of lactide is well ...
example 2
Polylactide-Doxorubicin Polymer (Doxo-PLA)
[0140]The catalyst (BDI)MgN(TMS)2 and D,L-lactide were treated as in Ptxl-PLA polymerization. The polymerization was conducted in a glove box. All the reaction vessels were covered with aluminum foil and the box light was turned off. First, doxorubicin was dissolved in DMF and stirred for 10 min, until it completely dissolved. (BDI)MgN(SiMe3)2 was then added to and dissolved in THF. The doxorubicin and (BDI)MgN(SiMe3)2 solutions were mixed for 15-20 min, and the solution changed color from orange red to purple. On HPLC analysis, the peak associated with doxorubicin shifted, indicating that a complex of (BDI)MgN(SiMe3)2 and doxorubicin was formed. The UV detector was at 450 nm. D,L-lactide was dissolved in THF and added dropwise into the mixture of doxorubicin and (BDI)MgN(SiMe3)2 with rapid stirring. The reaction process was monitored by HPLC until all of the doxorubicin was gone. The UV spectrum of Doxo-LA exhibited an absorption at 325-400...
example 3
Dtxl-PLA using 1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD) or BDI—Mg—N(TMS)2
[0141]The TBD or BDI—Mg—N(TMS)2 catalyst was mixed with docetaxel and the lactide was added to the mixture of catalyst and initiator. For example, docetaxel and TBD were dissolved in THF solution and stirred for 5-10 min. (In HPLC, the peak of docetaxel shifted, indicating the TBD formed complex with docetaxel). D,L-Lactide was dissolved in THF solution and added dropwise into the mixture of docetaxel and TBD. The reaction was similar to that of paclitaxel-PLA, monitored by FTIR and HPLC. Polymerization initiated by docetaxel-Mg(II) complex was carried out in the same way as described above for paclitaxel. Nanoparticles were formed similarly to Ptxl-LA nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com