Preparation method of all-solid polymer electrolyte through in-situ ring opening polymerization of epoxy compound, and application of the all-solid polymer electrolyte in all-solid lithium battery
An all-solid-state polymer and epoxy-based compound technology, which is applied in non-aqueous electrolyte batteries, electrolyte battery manufacturing, electrolyte immobilization/gelation, etc., can solve the problems of high solid/solid interface impedance, poor rate and cycle performance And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
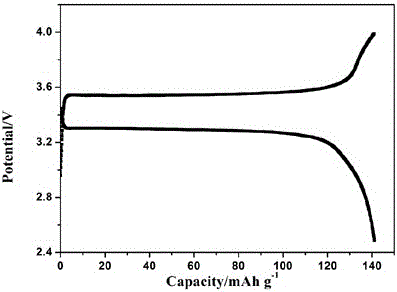
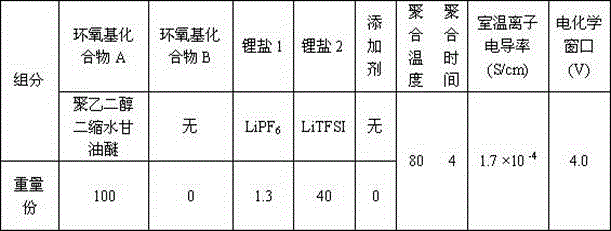
[0021] In a glove box filled with argon, LiTFSI, LiPF 6 Dissolve in polyethylene glycol diglycidyl ether monomer, and stir for 4 hours to mix evenly; inject the evenly mixed solution into Li / / SL (SL is a stainless steel pole piece), and place the SL / / SL battery at 80 o C for 4 hours, and then test the ionic conductivity and electrochemical stability window of the all-solid polymer electrolyte. Among them, polyethylene glycol diglycidyl ether and LiPF 6 , The mass ratio of LiTFSI is 100: 1.3: 40. The ratio of raw materials used to prepare solid polymer electrolytes is shown in the table, and the ion conductivity of the prepared polymer for lithium-ion batteries at room temperature is 1.7 × 10 -4 S / cm, the electrochemical window is 4.0V.
[0022] Table 1:
[0023]
Embodiment 2
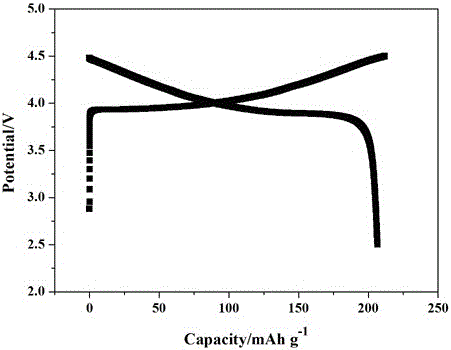
[0025] In a glove box filled with argon, LiTFSI, LiPF 6 Dissolve in polyethylene glycol diglycidyl ether monomer, add lithium lanthanum zirconium oxide nanoparticles, and then magnetically stir for 4 hours to mix evenly; inject the evenly mixed solution into Li / / SL (SL is a stainless steel pole piece), SL / / SL 30 in the battery o C for 4 hours, and then test the ionic conductivity and electrochemical stability window of the all-solid polymer electrolyte. Among them, polyethylene glycol diglycidyl ether and LiPF 6 The mass ratio of LiTFSI to lithium lanthanum zirconium oxide is 100:6:35:7. The ratio of raw materials used to prepare solid polymer electrolytes is shown in the table, and the ion conductivity of the prepared polymer for lithium-ion batteries at room temperature is 2.0 × 10 -4 S / cm, the electrochemical window is 4.8V.
[0026] Table 2:
[0027]
Embodiment 3
[0029] In a glove box filled with argon, LiTFSI, LiPF 6 Dissolve epoxy monomethoxypolyethylene glycol ether in polyethylene glycol diglycidyl ether monomer, add Al2O3 nanoparticles and then magnetically stir for 4 hours to mix evenly; inject the evenly mixed solution into Li / / SL (SL is a stainless steel pole piece), put 80 in SL / / SL battery o C for 4 hours, and then test the ionic conductivity and electrochemical stability window of the all-solid polymer electrolyte. Among them, polyethylene glycol diglycidyl ether and epoxy monomethoxypolyethylene glycol ether, LiPF 6 The mass ratio of , LiTFSI and aluminum oxide is 80 : 20 : 6 : 35 : 7. The ratio of raw materials used to prepare solid polymer electrolytes is shown in the table, and the ion conductivity of the prepared polymer for lithium-ion batteries at room temperature is 1.4 × 10 -4 S / cm, the electrochemical window is 4.8V.
[0030] table 3:
[0031]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com