Series salt of isopropoxy phenylguanidine and application thereof in preparing feed growth promoter
A technology of propoxybenzone and series salts, applied in the preparation of growth promoters for animal feed, in the field of series salts of propoxybenzone, achieving good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
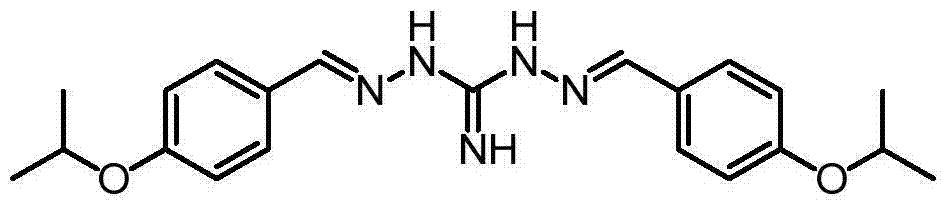
[0014] Embodiment 1: the preparation of free 2,2'-bis[[4-(isopropoxy)phenyl]methylene]-diaminoguanidine:
[0015]
[0016] Take 100g of 2,2'-bis[[4-(isopropoxy)phenyl]methylene]-diaminoguanidine hydrochloride (water content about 15%), add 750ml of 95% ethanol, add 12.5g of NaOH, 2, 2'-bis[[4-(isopropoxy)phenyl]methylene]-diaminoguanidine gradually dissolved and NaCl was precipitated. Stir for 0.5h and filter with suction. The filtrate was added dropwise to 3L of water, and a solid was precipitated. Suction filtration, the obtained solid was dried at 45 degrees to obtain 70g product (2,2'-bis[[4-(isopropoxy)phenyl]methylene]-diaminoguanidine).
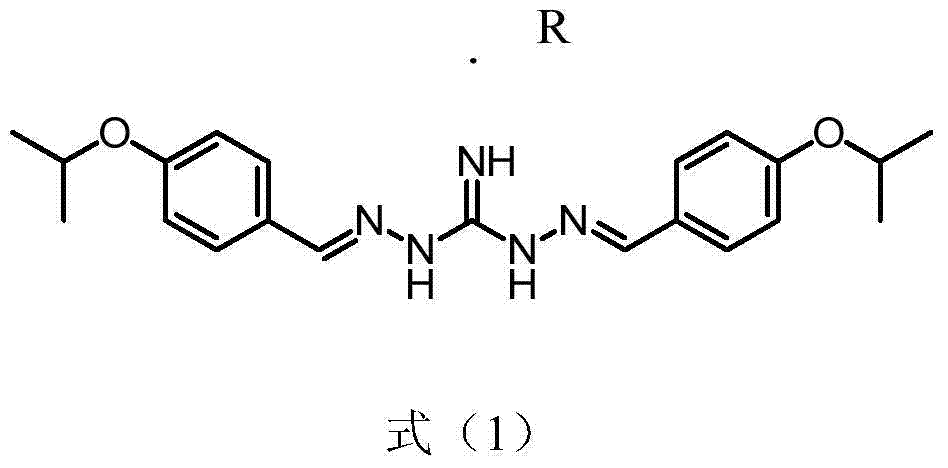
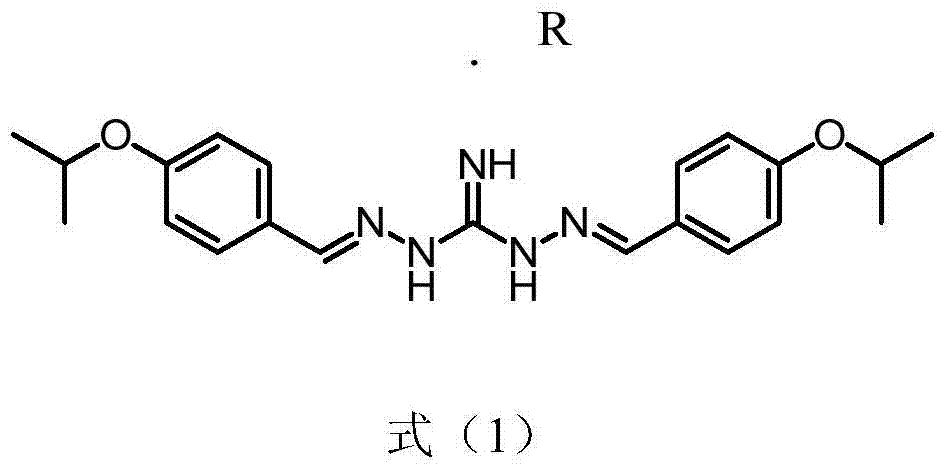
[0017] Preparation of 2,2'-bis[[4-(isopropoxy)phenyl]methylene]-diaminoguanidine.R salt as shown in formula (1)
[0018]
[0019] Wherein R is DL-lactic acid, methanesulfonic acid, 2-hydroxyethylsulfonic acid, citric acid, tartaric acid, benzoic acid, succinic acid, fumaric acid, maleic acid, acetic acid, sulfuric acid, phosph...
Embodiment 2
[0020] Example 2: 2,2'-bis[[4-(1-methylethoxy)phenyl]methylene]-carbonimidic dihydrazide.2-hydroxypropanoic acid / 2,2'-bis[[4-(isopropoxy)phenyl Preparation of ]methylene]-diaminoguanidine.DL-lactate
[0021] Take free 2,2'-bis[[4-(isopropoxy)phenyl]methylene]-diaminoguanidine 1.6g, add 8ml 95% ethanol, stir at room temperature, add 0.4g DL-lactate , stirred for 2h, filtered, the filter cake was washed with 95% ethanol, washed with water, then dried overnight at 45°C to obtain 1.1g product (2,2'-bis[[4-(isopropoxy)phenyl]methylene] -Diaminoguanidine. DL-lactate, or guanidine lactate), yield 55%. 1 H NMR (500MHz,d-DMSO)δ7.99(s,2H),δ7.67(d,4H),δ6.92(d,4H),δ6.56(br,2H),δ4.63-4.67 (m,2H),δ4.01(q,1H),δ1.28(d,12H),δ1.22(d,3H).
Embodiment 3
[0022] Example 3: 2,2'-bis[[4-(1-methylethoxy)phenyl]methylene]-carbonimidic dihydrazide.methanesulfonic acid / 2,2'-bis[[4-(isopropoxy)phenyl]ethylene Methyl]-diaminoguanidine. Preparation of mesylate
[0023] Take free 2,2'-bis[[4-(isopropoxy)phenyl]methylene]-diaminoguanidine 1.6g, add 8ml 95% ethanol, stir at room temperature, add 0.4g methanesulfonic acid, stir 2h, filtered, the filter cake was washed with 95% ethanol, washed with water, and then dried overnight at 45°C to obtain 1.4g of product (2,2'-bis[[4-(isopropoxy)phenyl]methylene]-bis Aminoguanidine. Mesylate, or guanidine mesylate) with a yield of 70%. 1 H NMR (500MHz,d-DMSO)δ11.8(br,1H),δ8.39(s,2H),δ8.26(br,2H),δ7.85(d,4H),δ7.01(d ,4H),δ4.70-4.75(m,2H),δ2.41(s,3H),δ1.28(d,12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com