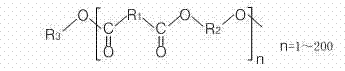
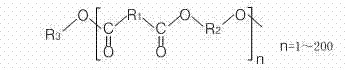
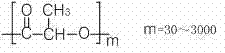Polylactic acid block copolymer
A technology of block copolymer and polylactic acid, which is applied in the field of polylactic acid block copolymer, can solve the problems of difficult preparation of polylactic acid block copolymer, long reaction time, irregular arrangement of chain segments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of block copolymers of polybutylene terephthalate (PBT) and polylactic acid (PLA).
[0041] Step (1). Ethanol triggers ring-opening polymerization of cyclic polybutylene terephthalate (CBT) to obtain single-end hydroxyl polybutylene terephthalate
[0042] Under nitrogen protection, 10gCBT, 0.5g ethanol and 0.105g Sn(Oct) 2 Put into the reaction bottle of 100ml that has been roasted over fire and has been cooled with nitrogen protection. Add 5ml of o-dichlorobenzene treated with anhydrous and anaerobic by syringe, so that the concentration of CBT is 2Kg / L, and the concentration of ethanol is 100g / L. The reaction was carried out at 180°C. After 10 minutes, the reaction was over (GPC monitoring showed no signal peak of CBT), cooled and filtered, and the reaction product was washed with anhydrous chloroform to wash away traces of unreacted CBT monomer. Vacuum at 80°C drying.
[0043] Step (2). Single-terminal hydroxyl PBT initiates lactide polymerization. The ...
Embodiment 2
[0053] Synthesis of block copolymers of polytrimethylene terephthalate (PTT) and polylactic acid (PLA).
[0054] Step (1). Methanol initiates ring-opening polymerization of cyclic polytrimethylene terephthalate (CTT) to obtain single-end hydroxyl polytrimethylene terephthalate
[0055] Under nitrogen protection, 10g CTT, 1.0g methanol and 0.0011g SnCl 2 Put into the reaction bottle of 100ml that has been roasted over fire and has been cooled with nitrogen protection. Add 5ml of tetrachloroethane treated with anhydrous and anaerobic by syringe, so that the concentration of CTT is 2Kg / L, and the concentration of methanol is 200g / L. The reaction was carried out at 180°C. After 5 minutes, the reaction was over (GPC monitoring showed no signal peak of CTT), cooled and filtered, and the reaction product was washed with anhydrous chloroform to wash away traces of unreacted CTT monomer. Vacuum at 60°C drying.
[0056] Step (2). Single-terminal hydroxyl PTT initiates lactide polymer...
Embodiment 3
[0066] Synthesis of block copolymers of polyethylene terephthalate (PET) and polylactic acid (PLA).
[0067] Step (1). 1-propanol initiates ring-opening polymerization of cyclic polyethylene terephthalate (CET) to obtain single-end hydroxyl polyethylene terephthalate
[0068] Under nitrogen protection, 10 g CET, 0.1 g 1-propanol and 0.0101 g SnBr 2 Put into the reaction bottle of 100ml that has been roasted over fire and has been cooled with nitrogen protection. Add 50ml of anhydrous and anaerobic treated nitrobenzene with a syringe, so that the concentration of CET is 0.2Kg / L, and the concentration of 1-propanol is 2g / L. The reaction was carried out at 120°C. After 60 minutes, the reaction was over (GPC monitoring had no signal peak of CET), cooled and filtered, and the reaction product was washed with anhydrous chloroform to wash away traces of unreacted CET monomers, and vacuumed at 80°C. drying.
[0069] Step (2). Single-end hydroxyl PET initiates lactide polymerization...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com