Stable formulations of polypeptides and uses thereof
a polypeptide and stable technology, applied in the field of single variable domain formulations, can solve the problems of short shelf life of liquid antibody preparations, physical instability, loss of biological activity of antibodies, etc., and achieve the effects of increasing the melting temperature and/or increasing the solubility and increasing the stability of single variable domains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation and Stability Studies with RANKL008a
example 1.1
Materials and Methods Used in the Study
[0676]1.1.1 Single variable domains
[0677]RANKL008a (SEQ ID NO: 4; EVQLVESGGGLVQPGGSLRLSCAASGFTFSSYPMGWFRQAPGKGREFVS SITGSGGSTYYADSVKGRFTISRDNAKNTLYLQMNSLRPEDTAVYYCAAYIRPDTYLSRDYRKYDYWGQGTLVTVSS GGGGSGGGSEVOLVESGGGLVQPGNSLRLSCAASGFTFSSFGMSWVRQAPGKGLEWVSSISGSGSDTLYADSVKG RFTISRDNAKTTLYLQMNSLRPEDTAVYYCTIGGSLSRSSQGTLVTVSSGGGGSGGGSEVQLVESGGGLVQPGGSLR LSCAASGFTFSSYPMGWFRQAPGKGREFVSSITGSGGSTYYADSVKGRFTISRDNAKNTLYLQMNSLRPEDTAVYYC AAYIRPDTYLSRDYRKYDYWGQGTLVTVSS) has been described as SEQ ID NO: 759 in WO 2008 / 142164. RANKL008a is a trivalent bispecific Nanobody consisting of three humanized variable domains of a heavy-chain llama antibody, of which two identical subunits are specific for binding to RANKL while the remaining subunit binds to HSA. The subunits are fused head-to-tail with a G / S linker in the following format: RANKL13H5-9GS-Alb8-9GS-RANKL13H5.
[0678]RANKL008a was expressed in Pichia pastoris and purified on SP Sepharose as a capturing step a...
example 1.2
Stability of the Nanobody in Different Buffers after Different Freeze / Thaw Cycles
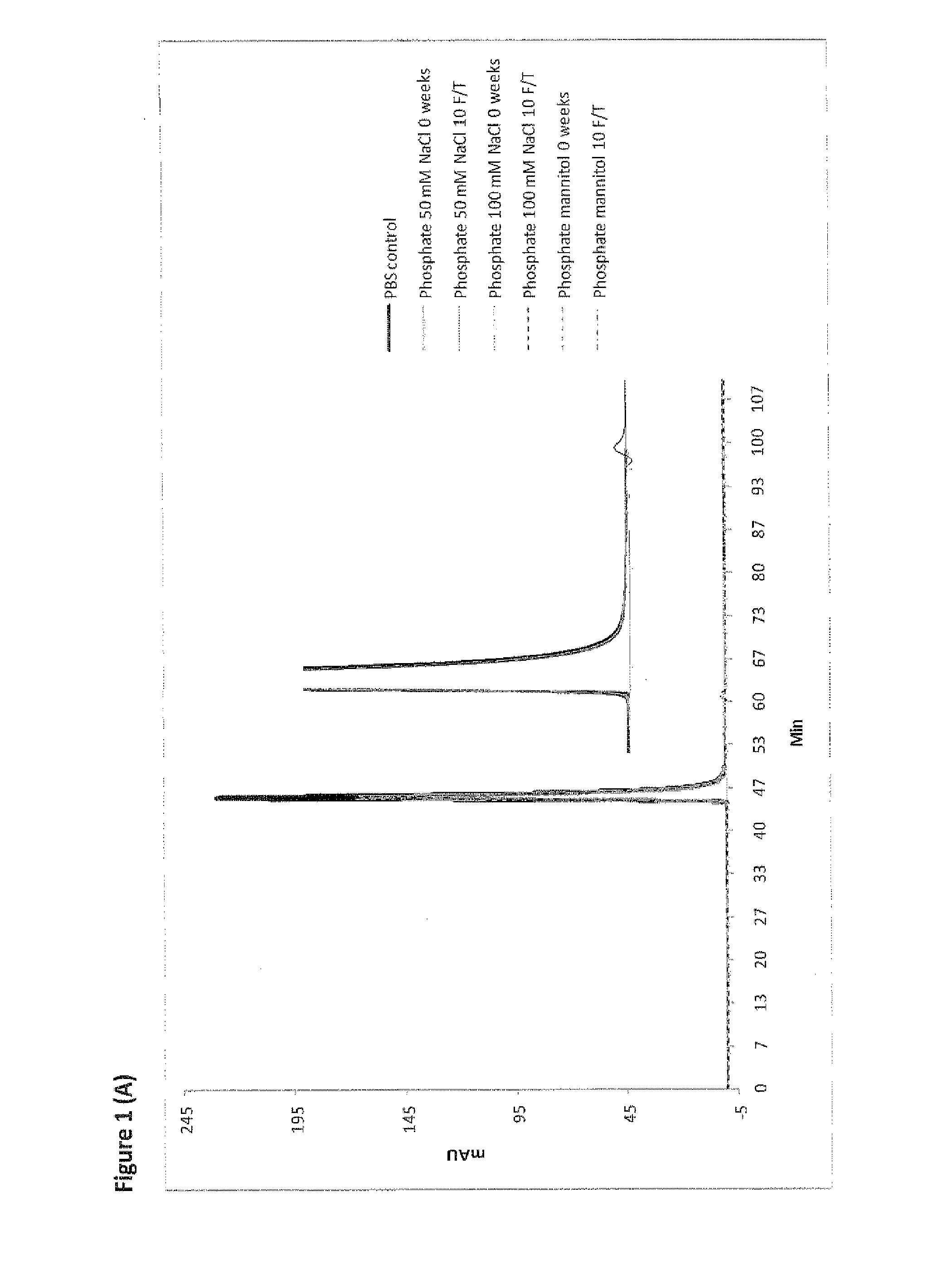
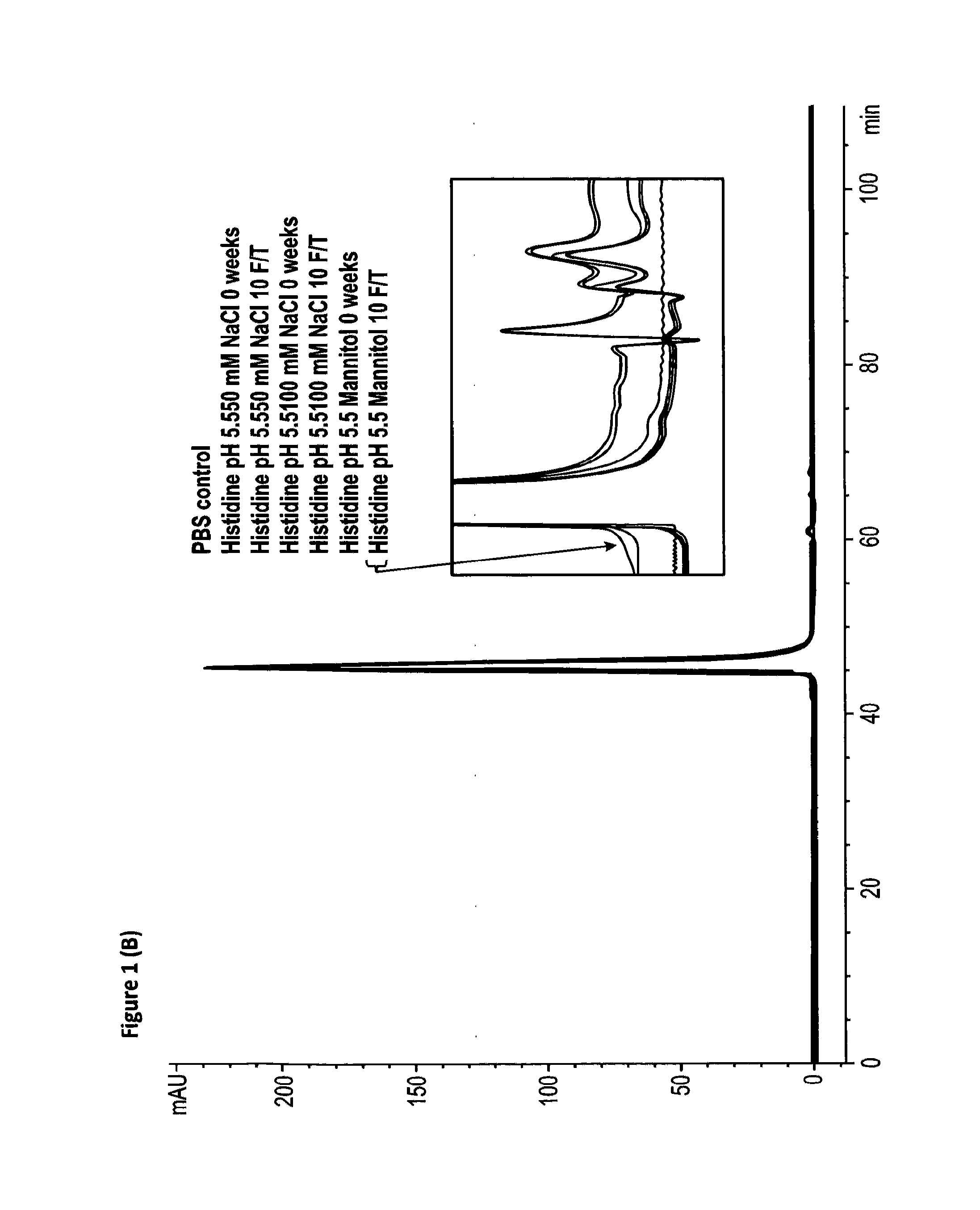
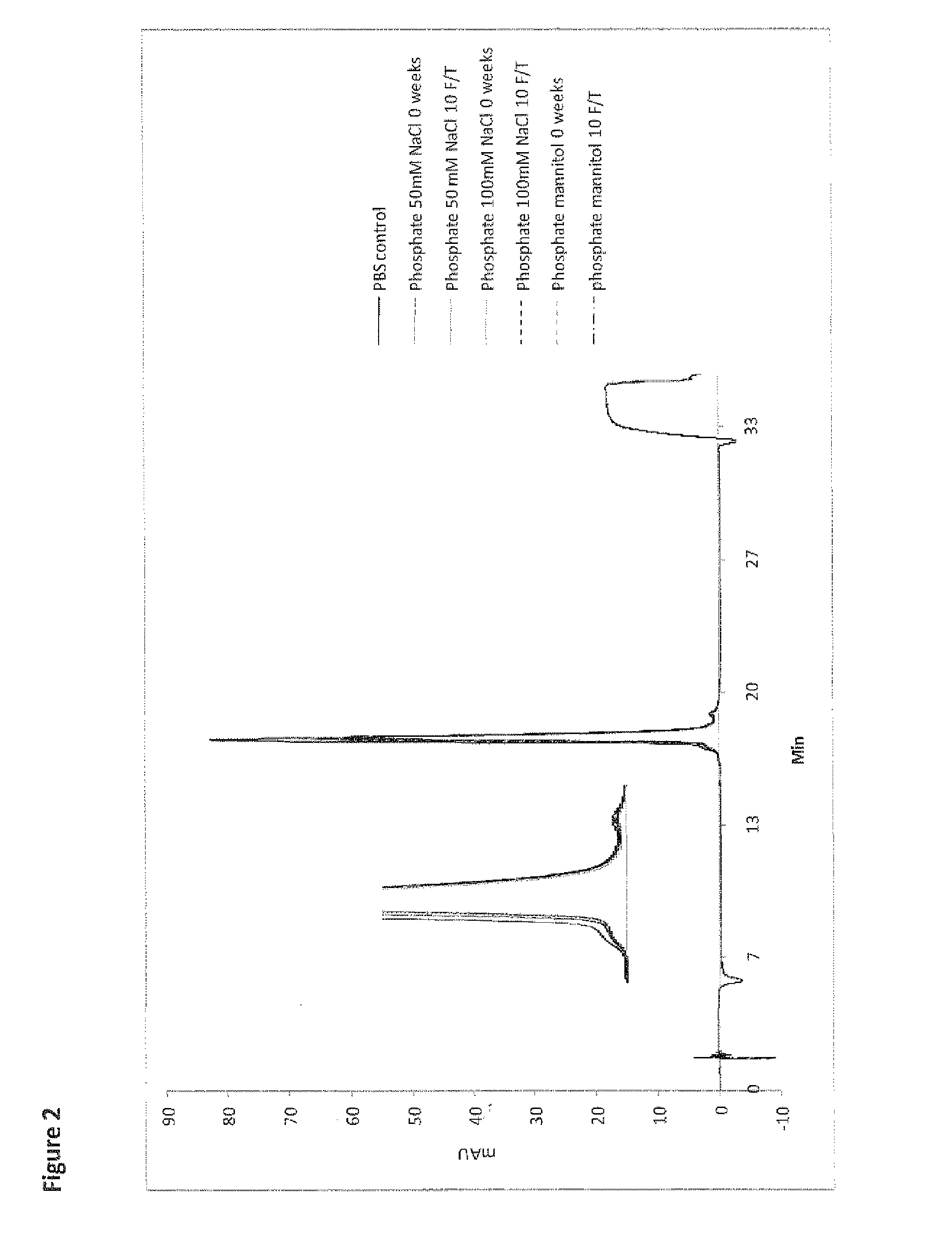
[0697]A freeze / thaw stability study was performed to determine the effect of repetitive freeze and thawing on the recovery, physical stability and chemical stability of RANKL008a. Aliquots of batch RANKL008a formulated at ˜60-85 mg / mL in the buffers 1-12 given in Table 1 were subjected to 10 freeze / thaw (F / T) cycles at −20° C. One F / T cycle is defined by freezing the sample for 1 hour in a freezer at −20° C. followed by thawing at room temperature for 30 minutes. The stressed samples were compared with reference material (stored at 4° C.) using SE-HPLC (FIG. 1 (A); representative figure of the experiments performed in phosphate buffer), RP-HPLC (FIG. 2; representative figure of the experiments performed in phosphate buffer) and the ELISA potency assays (Table 2). All other data of the freeze thaw experiments demonstrate similar patterns as given in FIGS. 1 and 2 (except FIG. 1 (B) see below).
[0698]Subje...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com