Novel dosage formulation
a technology of dosage formulation and dosage, applied in the field of new dosage formulation, can solve the problems of practical insolubility and achieve the effect of sufficient availability of active compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
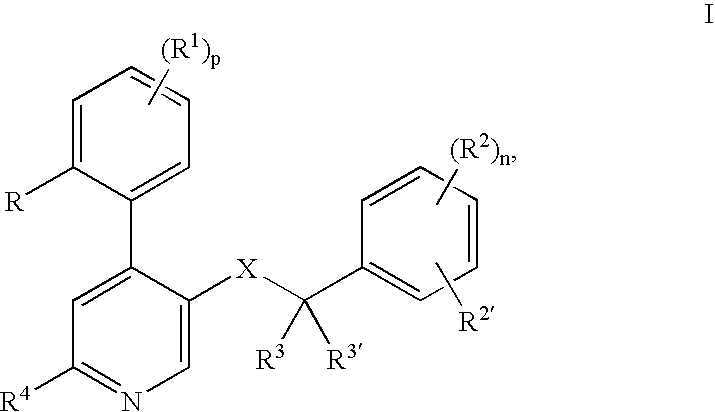
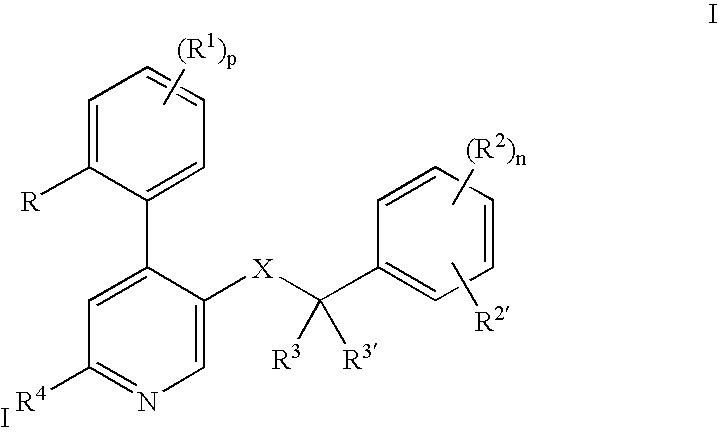
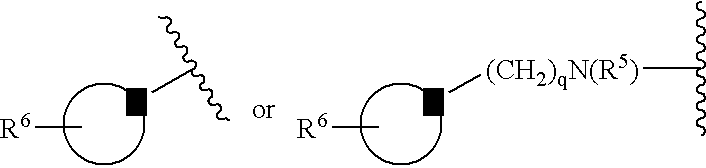
[0024] The following definitions of general terms used herein apply irrespective of whether the terms in question appear alone or in combination. It must be noted that, as used in the specification and the appended claims, the singular forms “a”, “an,” and “the” include plural forms unless the context clearly dictates otherwise.
[0025] The term “lower alkyl” denotes a straight- or branched-chain alkyl group containing from 1 to 7 carbon atoms. Nonlimiting examples of lower alkyl include methyl, ethyl, propyl, isopropyl, n-butyl, i-butyl, t-butyl, and the like.
[0026] The term “alkylene group” means a lower alkyl linker which is bound to a group at either end. Nonlimiting examples of alkylene groups include methylene, ethylene, propylene, and the like.
[0027] The term “lower alkoxy” denotes a alkyl group as defined above, which is attached through an oxygen atom. Nonlimiting examples of lower alkoxy groups include methoxy, ethoxy, propoxy, and the like.
[0028] The term “cycloalkyl” d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| inlet temperature | aaaaa | aaaaa |
| water soluble | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com